Dorsal Raphe Dopamine Neurons Signal Motivational Salience Dependent on Internal State, Expectation, and Behavioral Context
- PMID: 33563725
- PMCID: PMC8018733
- DOI: 10.1523/JNEUROSCI.2690-20.2021
Dorsal Raphe Dopamine Neurons Signal Motivational Salience Dependent on Internal State, Expectation, and Behavioral Context
Abstract
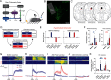
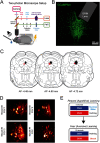
The ability to recognize motivationally salient events and adaptively respond to them is critical for survival. Here, we tested whether dopamine (DA) neurons in the dorsal raphe nucleus (DRN) contribute to this process in both male and female mice. Population recordings of DRNDA neurons during associative learning tasks showed that their activity dynamically tracks the motivational salience, developing excitation to both reward-paired and shock-paired cues. The DRNDA response to reward-predicting cues was diminished after satiety, suggesting modulation by internal states. DRNDA activity was also greater for unexpected outcomes than for expected outcomes. Two-photon imaging of DRNDA neurons demonstrated that the majority of individual neurons developed activation to reward-predicting cues and reward but not to shock-predicting cues, which was surprising and qualitatively distinct from the population results. Performing the same fear learning procedures in freely-moving and head-fixed groups revealed that head-fixation itself abolished the neural response to aversive cues, indicating its modulation by behavioral context. Overall, these results suggest that DRNDA neurons encode motivational salience, dependent on internal and external factors.SIGNIFICANCE STATEMENT Dopamine (DA) contributes to motivational control, composed of at least two functional cell types, one signaling for motivational value and another for motivational salience. Here, we demonstrate that DA neurons in the dorsal raphe nucleus (DRN) encode the motivational salience in associative learning tasks. Neural responses were dynamic and modulated by the animal's internal state. The majority of single-cells developed responses to reward or paired cues, but not to shock-predicting cues. Additional experiments with freely-moving and head-fixed mice showed that head-fixation abolished the development of cue responses during fear learning. This work provides further characterization on the functional roles of overlooked DRNDA populations and an example that neural responses can be altered by head-fixation, which is commonly used in neuroscience.
Keywords: dopamine; dorsal raphe nucleus; fiber photometry; head fixation; motivational salience; two-photon imaging.
Copyright © 2021 the authors.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
