Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin-streptomycin for wound dressing applications
- PMID: 33564036
- PMCID: PMC7873205
- DOI: 10.1038/s41598-021-82963-1
Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin-streptomycin for wound dressing applications
Abstract
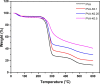
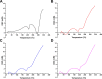
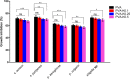
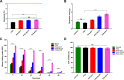
Hemorrhage is the major hindrance over the wound healing, which triggers microbial infections and might provoke traumatic death. Herein, new hemostatic and antibacterial PVA/Kaolin composite sponges were crosslinked using a freeze-thawing approach and boosted by penicillin-streptomycin (Pen-Strep). Physicochemical characteristics of developed membranes were analyzed adopting Fourier transformed infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), a thermal gravimetric analyzer (TGA), and differential scanning calorimetry (DSC). Furthermore, the impacts of kaolin concentrations on porosity, swelling behavior, gel fraction, and degradation of the membranes were investigated. SEM analyses revealed a spongy-like structure of hydrogels associated with high dispersion of kaolin inside PVA matrix. The thermal characteristics of PVA/Kaolin were significantly ameliorated compared to the prime PVA. Moreover, the results exhibited significant variations of swelling performance, surface roughness and pore capacity due to the alterations of kaolin contents. Besides, the adhesive strength ability was manifestly enhanced for PVA-K0.1 sponge. Biomedical evaluations including antibacterial activity, blood clotting index and thrombogenicity of the membranes were studied. The contact of PVA/Kaolin to blood revealed notable augmentation in blood clotting. Furthermore, the incorporation of kaolin into PVA presented mild diminution in antibacterial activities. Moreover, PVA/Kaolin composites illustrated no cellular toxicity towards fibroblast cells. These remarkable features substantiate that the PVA-K0.1 sponge could be applied as a multifunctional wound dressing.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Hermans MH. Wounds and ulcers: Back to the old nomenclature. Wounds Compendium Clin. Res. Pract. 2010;22:289–293. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

