Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data
- PMID: 33564135
- PMCID: PMC8182896
- DOI: 10.1038/s41433-020-01377-x
Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data
Abstract
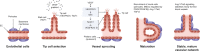
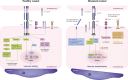
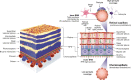
The angopoietin/tyrosine kinase with immunoglobulin and epidermal growth factor homology domains (Ang/Tie) pathway is an emerging key regulator in vascular development and maintenance. Its relevance to clinicians and basic scientists as a potential therapeutic target in retinal and choroidal vascular diseases is highlighted by recent preclinical and clinical evidence. The Ang/Tie pathway plays an important role in the regulation of vascular stability, in angiogenesis under physiological and pathological conditions, as well as in inflammation. Under physiological conditions, angiopoietin-1 (Ang-1) binds to and phosphorylates the Tie2 receptor, leading to downstream signalling that promotes cell survival and vascular stability. Angiopoietin-2 (Ang-2) is upregulated under pathological conditions and acts as a context-dependent agonist/antagonist of the Ang-1/Tie2 axis, causing vascular destabilisation and sensitising blood vessels to the effects of vascular endothelial growth factor-A (VEGF-A). Ang-2 and VEGF-A synergistically drive vascular leakage, neovascularisation and inflammation, key components of retinal vascular diseases. Preclinical evidence suggests that modulating the Ang/Tie pathway restores vascular stabilisation and reduces inflammation. This review discusses how targeting the Ang/Tie pathway or applying Ang-2/VEGF-A combination therapy may be a valuable therapeutic strategy for restoring vascular stability and reducing inflammation in the treatment of retinal and choroidal vascular diseases.
摘要: 本篇文章是关于不同照明条件对原发性开角型青光眼 (POAG) 患者视力和生活质量影响的研究的系统回顾。研究对CINAHL, MEDLINE, PsycARTICLES, PsycINFO, Embase and Ovid Nursing Database六个数据库进行系统文献检索, 截止发表日期为2019年4月。检索内容包括诊断为POAG的患者, 在变换照明设备/光照水平或炫光的情况下评估人群的视功能和生活质量。两名研究者独立筛选符合标准的受试者。从实验设计, 入选者标准, 结果与结论中挑选合格的研究并提取数据。入选研究的质量经过了严格的评估。在8437项研究中, 共有56项研究符合入选标准。在POAG患者中调查光照对以下因素的影响: 生活质量 (18/56),心理物理学干预 (16/56), 功能性视力 (10/56), 日常活动 (10/56) 和定性发现 (2/56)。POAG会影响患者的低亮度对比敏感度, 炫光症状, 暗适应的时间和程度。在视觉生活质量调查问卷中, 根据POAG患者反馈, 照明设备, 炫光和暗适应的问题较其它问题更多见。这些问题随着进行性视野的缺失而严重, 与同年龄对照组相比(AMC), POAG患者在发病的早期在低亮度和不同亮度切换的环境中会面临更多的困难, 这对之前POAG患者早期阶段没有症状的认知进行了挑战。但是, 基于性能方面的研究很少显示POAG参与者和AMC在模拟非最佳照明条件下日常活动方面有显着差异。 需要对较大的样本进行进一步研究, 以优化环境照明和面向任务的照明, 以支持患者适应POAG。未来亟待大样本的研究为POAG患者提供优化环境和适合工作的照明。.
Conflict of interest statement
AMJ is a consultant for Allergan, Bayer, Novartis and Roche, and has received research funding from Bayer and Novartis. FR is a consultant for Allergan, Bayer, Chengdu Kanghong, Iveric Bio, Formicon, Genentech/Roche, MS&D and Novartis. LPP and CK are employees of F. Hoffmann-La Roche Ltd. CQR is an employee of Genentech, Inc., and holds stock options in Genentech, Inc. MZ is a consultant for Cell Cure, Chengdu Kanghong, Frequency Therapeutics, Genentech/Roche, Iduna, Iveric Bio, Life Biosciences, Novartis Pharma AG, NVasc, Ophthotech, Perfuse Therapeutics and Selphagy.
Figures

References
-
- Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017;16:635–61. - PubMed
-
- Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. - PubMed
-
- Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. - PubMed
-
- Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti–vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4:19–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

