Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization
- PMID: 33564154
- PMCID: PMC8221230
- DOI: 10.1038/s41580-021-00328-y
Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization
Erratum in
-
Publisher Correction: Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization.Nat Rev Mol Cell Biol. 2021 Apr;22(4):299. doi: 10.1038/s41580-021-00353-x. Nat Rev Mol Cell Biol. 2021. PMID: 33608692 No abstract available.
Abstract
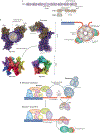
The regulation of telomere length in mammals is crucial for chromosome end-capping and thus for maintaining genome stability and cellular lifespan. This process requires coordination between telomeric protein complexes and the ribonucleoprotein telomerase, which extends the telomeric DNA. Telomeric proteins modulate telomere architecture, recruit telomerase to accessible telomeres and orchestrate the conversion of the newly synthesized telomeric single-stranded DNA tail into double-stranded DNA. Dysfunctional telomere maintenance leads to telomere shortening, which causes human diseases including bone marrow failure, premature ageing and cancer. Recent studies provide new insights into telomerase-related interactions (the 'telomere replisome') and reveal new challenges for future telomere structural biology endeavours owing to the dynamic nature of telomere architecture and the great number of structures that telomeres form. In this Review, we discuss recently determined structures of the shelterin and CTC1-STN1-TEN1 (CST) complexes, how they may participate in the regulation of telomere replication and chromosome end-capping, and how disease-causing mutations in their encoding genes may affect specific functions. Major outstanding questions in the field include how all of the telomere components assemble relative to each other and how the switching between different telomere structures is achieved.
Conflict of interest statement
Competing interests
T.R.C. is on the board of directors of Merck and a consultant for STORM Therapeutics and Eikon Therapeutics.
Figures
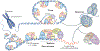


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

