Total synthesis of decarboxyaltenusin
- PMID: 33564332
- PMCID: PMC7849237
- DOI: 10.3762/bjoc.17.22
Total synthesis of decarboxyaltenusin
Abstract
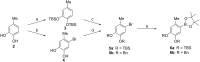
The total synthesis of decarboxyaltenusin (5'-methoxy-6-methyl-[1,1'-biphenyl]-3,3',4-triol), a toxin produced by various mold fungi, has been achieved in seven steps in a yield of 31% starting from 4-methylcatechol and 1-bromo-3,5-dimethoxybenzene, where the longest linear sequence consists of five steps. The key reaction was a palladium-catalyzed Suzuki coupling of an aromatic boronate with a brominated resorcin derivative.
Keywords: Suzuki coupling; biaryls; boronates; mycotoxins; polyketides.
Copyright © 2021, Warmuth et al.
Figures



References
-
- Kameda K, Aoki H, Namiki M, Overeem J C. Tetrahedron Lett. 1974;15(1):103–106. doi: 10.1016/s0040-4039(01)82147-x. - DOI
-
- Wang M, Li X, Li C, Wang B. Haiyang Kexue. 2014;38:1–5. doi: 10.11759/hykx20130323002. Chem. Abstr.2015,162, 652122. - DOI
-
- Guo L-z, Liu C-x, Xu B, Chen J-f, Zou K, Guo Z-y. Huaxue Yanjiu Yu Yingyong. 2014;26:306–310. Chem. Abstr.2014,162, 445682.
LinkOut - more resources
Full Text Sources
Other Literature Sources
