This is a preprint.
SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection
- PMID: 33564773
- PMCID: PMC7872365
- DOI: 10.1101/2021.02.02.21250988
SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection
Update in
-
SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection.Nat Commun. 2021 Jul 29;12(1):4678. doi: 10.1038/s41467-021-24938-4. Nat Commun. 2021. PMID: 34326343 Free PMC article.
Abstract
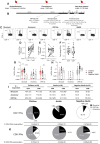
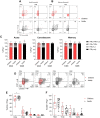
SARS-CoV-2 infection of children leads to a mild illness and the immunological differences with adults remains unclear. We quantified the SARS-CoV-2 specific T cell responses in adults and children (<13 years of age) with RT-PCR confirmed asymptomatic and symptomatic infection for long-term memory, phenotype and polyfunctional cytokines. Acute and memory CD4+ T cell responses to structural SARS-CoV-2 proteins significantly increased with age, whilst CD8+ T cell responses increased with time post infection. Infected children had significantly lower CD4+ and CD8+ T cell responses to SARS-CoV-2 structural and ORF1ab proteins compared to infected adults. SARS-CoV-2-specific CD8+ T cell responses were comparable in magnitude to uninfected negative adult controls. In infected adults CD4+ T cell specificity was skewed towards structural peptides, whilst children had increased contribution of ORF1ab responses. This may reflect differing T cell compartmentalisation for antigen processing during antigen exposure or lower recruitment of memory populations. T cell polyfunctional cytokine production was comparable between children and adults, but children had a lower proportion of SARS-CoV-2 CD4+ T cell effector memory. Compared to adults, children had significantly lower levels of antibodies to β-coronaviruses, indicating differing baseline immunity. Total T follicular helper responses was increased in children during acute infection indicating rapid co-ordination of the T and B cell responses. However total monocyte responses were reduced in children which may be reflective of differing levels of inflammation between children and adults. Therefore, reduced prior β-coronavirus immunity and reduced activation and recruitment of de novo responses in children may drive milder COVID-19 pathogenesis.
Keywords: COVID19; SARS-CoV-2; T cells; asymptomatic; common cold coronavirus; paediatric.
Conflict of interest statement
Competing interests None to declare.
Figures


References
-
- ADDETIA A., CRAWFORD K. H. D., DINGENS A., ZHU H., ROYCHOUDHURY P., HUANG M. L., JEROME K. R., BLOOM J. D. & GRENINGER A. L. 2020. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J Clin Microbiol, 58. - PMC - PubMed
-
- ANDERSON E. M., GOODWIN E. C., VERMA A., AREVALO C. P., BOLTON M. J., WEIRICK M. E., GOUMA S., MCALLISTER C. M., CHRISTENSEN S. R., WEAVER J., HICKS P., MANZONI T. B., ONIYIDE O., RAMAGE H., MATHEW D., BAXTER A. E., OLDRIDGE D. A., GREENPLATE A. R., WU J. E., ALANIO C., D’ANDREA K., KUTHURU O., DOUGHERTY J., PATTEKAR A., KIM J., HAN N., APOSTOLIDIS S. A., HUANG A. C., VELLA L. A., WHERRY E. J., MEYER N. J., CHERRY S., BATES P., RADER D. J. & HENSLEY S. E. 2020. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. medRxiv. - PMC - PubMed
-
- BACHER P., ROSATI E., ESSER D., MARTINI G. R., SAGGAU C., SCHIMINSKY E., DARGVAINIENE J., SCHRODER I., WIETERS I., KHODAMORADI Y., EBERHARDT F., VEHRESCHILD M., NEB H., SONNTAGBAUER M., CONRAD C., TRAN F., ROSENSTIEL P., MARKEWITZ R., WANDINGER K. P., AUGUSTIN M., RYBNIKER J., KOCHANEK M., LEYPOLDT F., CORNELY O. A., KOEHLER P., FRANKE A. & SCHEFFOLD A. 2020. Low-Avidity CD4(+) T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity, 53, 1258–1271 e5. - PMC - PubMed
-
- BLANCO-MELO D., NILSSON-PAYANT B. E., LIU W. C., UHL S., HOAGLAND D., MOLLER R., JORDAN T. X., OISHI K., PANIS M., SACHS D., WANG T. T., SCHWARTZ R. E., LIM J. K., ALBRECHT R. A. & TENOEVER B. R. 2020. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell, 181, 1036–1045 e9. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
