This is a preprint.
Clinical and Economic Impact of Widespread Rapid Testing to Decrease SARS-CoV-2 Transmission
- PMID: 33564779
- PMCID: PMC7872371
- DOI: 10.1101/2021.02.06.21251270
Clinical and Economic Impact of Widespread Rapid Testing to Decrease SARS-CoV-2 Transmission
Update in
-
Clinical and Economic Effects of Widespread Rapid Testing to Decrease SARS-CoV-2 Transmission.Ann Intern Med. 2021 Jun;174(6):803-810. doi: 10.7326/M21-0510. Epub 2021 Mar 9. Ann Intern Med. 2021. PMID: 33683930 Free PMC article.
Abstract
Background: The value of frequent, rapid testing to reduce community transmission of SARS-CoV-2 is poorly understood.
Objective: To define performance standards and predict the clinical, epidemiological, and economic outcomes of nationwide, home-based, antigen testing.
Design: A simple compartmental epidemic model estimated viral transmission, clinical history, and resource use, with and without testing.
Data sources: Parameter values and ranges informed by Centers for Disease Control guidance and published literature.
Target population: United States population.
Time horizon: 60 days.
Perspective: Societal. Costs include: testing, inpatient care, and lost workdays.
Intervention: Home-based SARS-CoV-2 antigen testing.
Outcome measures: Cumulative infections and deaths, numbers isolated and/or hospitalized, and total costs.
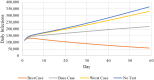
Results of base-case analysis: Without a testing intervention, the model anticipates 15 million infections, 125,000 deaths, and $10.4 billion in costs ($6.5 billion inpatient; $3.9 billion lost productivity) over a 60-day horizon. Weekly availability of testing may avert 4 million infections and 19,000 deaths, raising costs by $21.5 billion. Lower inpatient outlays ($5.9 billion) would partially offset additional testing expenditures ($12.0 billion) and workdays lost ($13.9 billion), yielding incremental costs per infection (death) averted of $5,400 ($1,100,000).
Results of sensitivity analysis: Outcome estimates vary widely under different behavioral assumptions and testing frequencies. However, key findings persist across all scenarios: large reductions in infections, mortality, and hospitalizations; and costs per death averted roughly an order of magnitude lower than commonly accepted willingness-to-pay values per statistical life saved ($5-17 million).
Limitations: Analysis restricted to at-home testing and limited by uncertainties about test performance.
Conclusion: High-frequency home testing for SARS-CoV-2 using an inexpensive, imperfect test could contribute to pandemic control at justifiable cost and warrants consideration as part of a national containment strategy.
Figures


References
-
- Atkenson A, Droste M, Mina M, Stock J. Economic benefits of COVID-19 screening tests. medRxiv 2020.10.22.20217984; doi: 10.1101/2020.10.22.20217984 - DOI
-
- Rapid Tests. Expert Letter. 15 December 2020. Accessed at www.rapidtests.org/expert-letter on 21 January 2021.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
