This is a preprint.
Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics
- PMID: 33564787
- PMCID: PMC7872382
- DOI: 10.1101/2021.02.01.21250946
Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics
Update in
-
Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics.BMC Infect Dis. 2022 Mar 25;22(1):284. doi: 10.1186/s12879-022-07285-7. BMC Infect Dis. 2022. PMID: 35337266 Free PMC article.
Abstract
There is an urgent need to expand testing for SARS-CoV-2 and other respiratory pathogens as the global community struggles to control the COVID-19 pandemic. Current diagnostic methods can be affected by supply chain bottlenecks and require the assistance of medical professionals, impeding the implementation of large-scale testing. Self-collection of saliva may solve these problems, as it can be completed without specialized training and uses generic materials. In this study, we observed thirty individuals who self-collected saliva using four different collection devices and analyzed their feedback. Two of these devices, a funnel and bulb pipette, were used to evaluate at-home saliva collection by 60 individuals. All devices enabled the safe, unsupervised self-collection of saliva. The quantity and quality of the samples received were acceptable for SARS-CoV-2 diagnostic testing, as determined by RNase P detection. Here, we demonstrate inexpensive, generic, buffer free collection devices suitable for unsupervised and home saliva self-collection.
Conflict of interest statement
Declaration of interest N.D.G. is a paid consultant for Tempus. The remaining authors declare no competing interests.
Figures
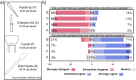

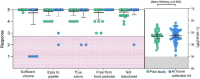
References
-
- Adamopoulou M., Vairaktaris E., Panis V., Nkenke E., Neukam F.W., and Yapijakis C. (2008). HPV detection rate in saliva may depend on the immune system efficiency. In Vivo 22, 599–602. - PubMed
-
- FDA (2018). FDA authorizes first test to aid in detecting a type of herpes virus in newborns called cytomegalovirus.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
