Coronavirus Disease 2019 (COVID-19) Diagnostic Clinical Decision Support: A Pre-Post Implementation Study of CORAL (COvid Risk cALculator)
- PMID: 33564833
- PMCID: PMC7929052
- DOI: 10.1093/cid/ciab111
Coronavirus Disease 2019 (COVID-19) Diagnostic Clinical Decision Support: A Pre-Post Implementation Study of CORAL (COvid Risk cALculator)
Abstract
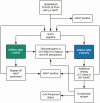
Background: Isolation of hospitalized persons under investigation (PUIs) for coronavirus disease 2019 (COVID-19) reduces nosocomial transmission risk. Efficient evaluation of PUIs is needed to preserve scarce healthcare resources. We describe the development, implementation, and outcomes of an inpatient diagnostic algorithm and clinical decision support system (CDSS) to evaluate PUIs.
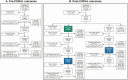
Methods: We conducted a pre-post study of CORAL (COvid Risk cALculator), a CDSS that guides frontline clinicians through a risk-stratified COVID-19 diagnostic workup, removes transmission-based precautions when workup is complete and negative, and triages complex cases to infectious diseases (ID) physician review. Before CORAL, ID physicians reviewed all PUI records to guide workup and precautions. After CORAL, frontline clinicians evaluated PUIs directly using CORAL. We compared pre- and post-CORAL frequency of repeated severe acute respiratory syndrome coronavirus 2 nucleic acid amplification tests (NAATs), time from NAAT result to PUI status discontinuation, total duration of PUI status, and ID physician work hours, using linear and logistic regression, adjusted for COVID-19 incidence.
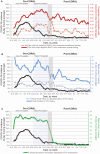
Results: Fewer PUIs underwent repeated testing after an initial negative NAAT after CORAL than before CORAL (54% vs 67%, respectively; adjusted odd ratio, 0.53 [95% confidence interval, .44-.63]; P < .01). CORAL significantly reduced average time to PUI status discontinuation (adjusted difference [standard error], -7.4 [0.8] hours per patient), total duration of PUI status (-19.5 [1.9] hours per patient), and average ID physician work-hours (-57.4 [2.0] hours per day) (all P < .01). No patients had a positive NAAT result within 7 days after discontinuation of precautions via CORAL.
Conclusions: CORAL is an efficient and effective CDSS to guide frontline clinicians through the diagnostic evaluation of PUIs and safe discontinuation of precautions.
Keywords: COVID-19 diagnosis; clinical decision support system; diagnostic algorithm; electronic health record.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Hanson KE, Caliendo AM, Arias CA, et al. . Infectious Diseases Society of America guidelines on the diagnosis of COVID-19. Infectious Diseases Society of America, 2020. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnost.... Accessed 5 July 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

