Switching to iGlarLixi versus continuation of a daily or weekly glucagon-like peptide-1 receptor agonist (GLP-1 RA) in insufficiently controlled type 2 diabetes: A LixiLan-G trial subgroup analysis by HbA1c and GLP-1 RA use at screening
- PMID: 33565209
- PMCID: PMC8252076
- DOI: 10.1111/dom.14345
Switching to iGlarLixi versus continuation of a daily or weekly glucagon-like peptide-1 receptor agonist (GLP-1 RA) in insufficiently controlled type 2 diabetes: A LixiLan-G trial subgroup analysis by HbA1c and GLP-1 RA use at screening
Abstract
Aim: In people with type 2 diabetes (T2D) requiring intensification beyond glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and oral antihyperglycaemic drugs (OADs), switching to iGlarLixi was shown to be efficacious and well-tolerated in the LixiLan-G trial. This exploratory analysis of LixiLan-G assessed the efficacy and safety of switching to iGlarLixi versus continuing GLP-1 RA therapy, stratified by screening HbA1c level (≥7.0 to ≤7.5 %; >7.5 to ≤8.0 %; >8.0 to ≤9.0 % [≥53 to ≤58 mmol/mol; >58 to ≤64 mmol/mol; >64 to ≤75 mmol/mol]) and previous GLP-1 RA regimen at screening (once/twice daily or once weekly).
Materials and methods: Endpoints for all subgroups included: change in HbA1c, achievement of HbA1c <7 % and hypoglycaemia events. Adverse events and changes in fasting plasma glucose (FPG), 2-hour postprandial plasma glucose (PPG), 2-hour PPG excursion and weight were analysed according to previous GLP-1 RA regimen.
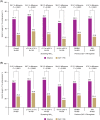
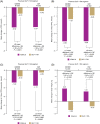
Results: Switching to iGlarLixi in all subgroups resulted in significantly greater reductions in HbA1c and proportions of participants reaching HbA1c <7 % (including with no documented hypoglycaemia) at Week 26 compared with continued GLP-1 RA treatment. Switching to iGlarLixi also led to significantly greater reductions in FPG, 2-hour PPG, and 2-hour PPG excursion, irrespective of previous GLP-1 RA regimen. Rates of hypoglycaemia were low, but slightly higher in those who switched to iGlarLixi for all subgroups. Modest weight gain was seen with iGlarLixi, irrespective of previous GLP-1 RA regimen.
Conclusions: Switching to iGlarLixi improved glycaemic control, regardless of screening HbA1c or previous GLP-1 RA type, offering a simple, efficacious and well-tolerated treatment intensification option for people with T2D inadequately controlled by GLP-1 RAs and OADs.
Keywords: GLP-1 analogue; basal insulin; glycaemic control; incretin therapy; insulin therapy; type 2 diabetes.
© 2021 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
J.R. has been a consultant for Applied Therapeutics, Boehringer Ingelheim, Eli Lilly, Intarcia, Janssen, Novo Nordisk, Oramed and Sanofi, and has received grant/research support from Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genentech, GlaxoSmithKline, Intarcia, Janssen, Lexicon, Merck, Novo Nordisk, Oramed, Pfizer and Sanofi. L.B. has been a consultant for AstraZeneca, Gilead Sciences, Janssen, Merck, Novo Nordisk and Sanofi, has received grant/research support (including to his institution) from Janssen, Lexicon, Merck, Novo Nordisk and Sanofi, and has been a speaker for Janssen, Novo Nordisk and Sanofi. V.R.A. has received clinical trial/research support from Applied Therapeutics, Fractyl/Premier, Novo Nordisk and Sanofi, has been a consultant for Applied Therapeutics, Novo Nordisk and Sanofi, and her spouse is an employee of Janssen. J.F. has been a consultant for Boehringer Ingelheim, Johnson & Johnson, Eli Lilly, Merck, Novo Nordisk and Sanofi, has received grant/research support from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Novo Nordisk, Pfizer, Sanofi and Theracos, and has been a speaker for Merck and Sanofi. E.S., C.J. and E.N. are employees of Sanofi. S.D.P. has received grant/research support from AstraZeneca, Boehringer Ingelheim, Merck and Novartis, and honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Mundipharma, Novartis, Novo Nordisk, Sanofi, Servier and Takeda.
Figures

Similar articles
-
Switching to iGlarLixi Versus Continuing Daily or Weekly GLP-1 RA in Type 2 Diabetes Inadequately Controlled by GLP-1 RA and Oral Antihyperglycemic Therapy: The LixiLan-G Randomized Clinical Trial.Diabetes Care. 2019 Nov;42(11):2108-2116. doi: 10.2337/dc19-1357. Epub 2019 Sep 17. Diabetes Care. 2019. PMID: 31530665 Clinical Trial.
-
Impact of disease duration and β-cell reserve on the efficacy of switching to iGlarLixi in adults with type 2 diabetes on glucagon-like peptide-1 receptor agonist therapy: Exploratory analyses from the LixiLan-G trial.Diabetes Obes Metab. 2020 Sep;22(9):1567-1576. doi: 10.1111/dom.14068. Epub 2020 May 28. Diabetes Obes Metab. 2020. PMID: 32323437 Free PMC article. Clinical Trial.
-
iGlarLixi provides improved early glycaemic control after 12 weeks of treatment compared with basal insulin in Asian people with type 2 diabetes: A post hoc analysis of the LixiLan-O-AP and LixiLan-L-CN studies.Diabetes Obes Metab. 2025 May;27(5):2593-2600. doi: 10.1111/dom.16260. Epub 2025 Feb 27. Diabetes Obes Metab. 2025. PMID: 40013432 Free PMC article. Clinical Trial.
-
What is the 'real-world' experience with fixed-ratio combination therapy (insulin + GLP-1 receptor agonist) in routine clinical practice? Take-home messages for clinicians regarding key outcomes.Diabetes Obes Metab. 2025 Aug;27 Suppl 7(Suppl 7):26-41. doi: 10.1111/dom.16593. Epub 2025 Jul 10. Diabetes Obes Metab. 2025. PMID: 40637050 Free PMC article. Review.
-
Titratable fixed-ratio combination of insulin glargine plus lixisenatide: A simplified approach to glycemic control in type 2 diabetes mellitus.Diabetes Res Clin Pract. 2020 Dec;170:108478. doi: 10.1016/j.diabres.2020.108478. Epub 2020 Sep 28. Diabetes Res Clin Pract. 2020. PMID: 33002548 Review.
References
-
- American Diabetes Association . 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes ‐ 2020. Diabetes Care. 2020;43(Suppl 1):S98‐S110. - PubMed
-
- Rosenstock J, Aronson R, Grunberger G, et al. Benefits of lixilan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled with oral agents: The LixiLan‐O randomized trial. Diabetes Care. 2016;39(11):2026‐2035. - PubMed
-
- Aroda V, Rosenstock J, Wysham C, et al. Efficacy and safety of lixilan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: The LixiLan‐L randomized trial. Diabetes Care. 2016;39:1972‐1980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

