YAP expression in endothelial cells prevents ventilator-induced lung injury
- PMID: 33565367
- PMCID: PMC8238153
- DOI: 10.1152/ajplung.00472.2020
YAP expression in endothelial cells prevents ventilator-induced lung injury
Abstract
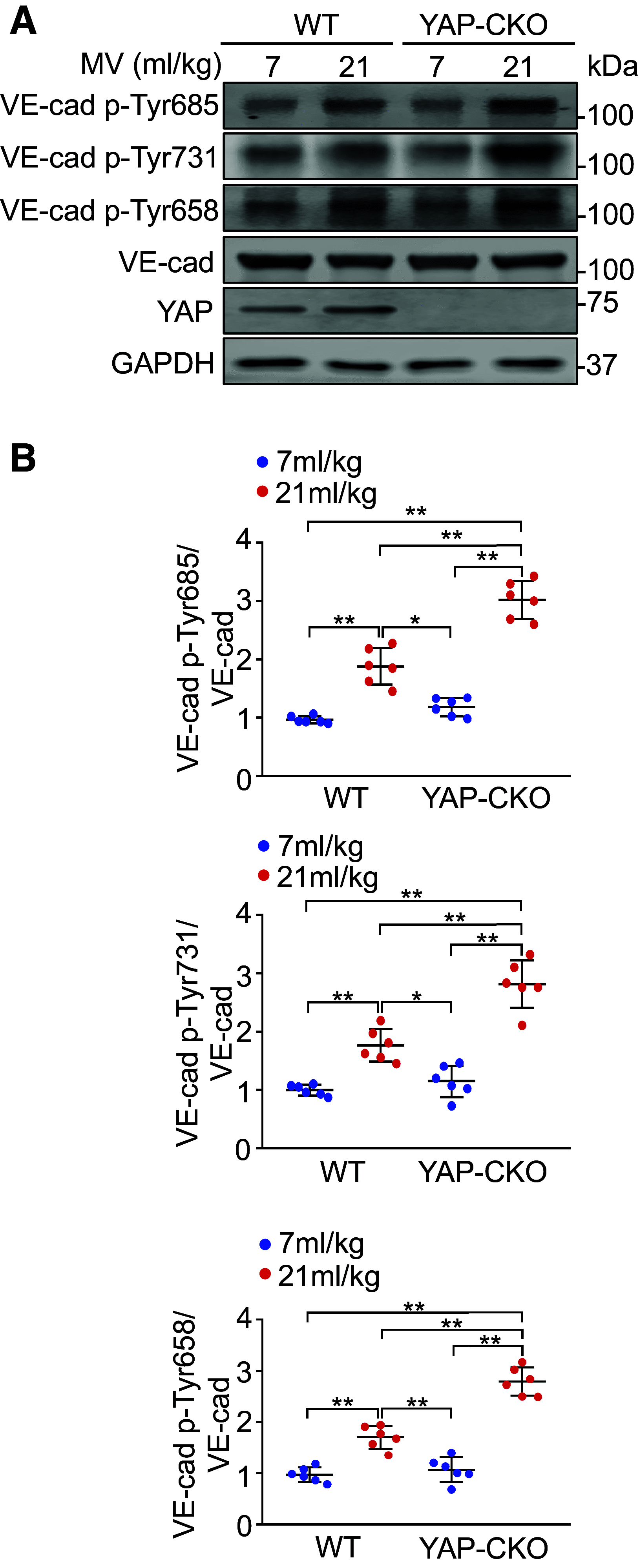
Ventilator-induced lung injury is associated with an increase in mortality in patients with respiratory dysfunction, although mechanical ventilation is an essential intervention implemented in the intensive care unit. Intrinsic molecular mechanisms for minimizing lung inflammatory injury during mechanical ventilation remain poorly defined. We hypothesize that Yes-associated protein (YAP) expression in endothelial cells protects the lung against ventilator-induced injury. Wild-type and endothelial-specific YAP-deficient mice were subjected to a low (7 mL/kg) or high (21 mL/kg) tidal volume (VT) ventilation for 4 h. Infiltration of inflammatory cells into the lung, vascular permeability, lung histopathology, and the levels of inflammatory cytokines were measured. Here, we showed that mechanical ventilation with high VT upregulated YAP protein expression in pulmonary endothelial cells. Endothelial-specific YAP knockout mice following high VT ventilation exhibited increased neutrophil counts and protein content in bronchoalveolar lavage fluid, Evans blue leakage, and histological lung injury compared with wild-type littermate controls. Deletion of YAP in endothelial cells exaggerated vascular endothelial (VE)-cadherin phosphorylation, downregulation of vascular endothelial protein tyrosine phosphatase (VE-PTP), and dissociation of VE-cadherin and catenins following mechanical ventilation. Importantly, exogenous expression of wild-type VE-PTP in the pulmonary vasculature rescued YAP ablation-induced increases in neutrophil counts and protein content in bronchoalveolar lavage fluid, vascular leakage, and histological lung injury as well as VE-cadherin phosphorylation and dissociation from catenins following ventilation. These data demonstrate that YAP expression in endothelial cells suppresses lung inflammatory response and edema formation by modulating VE-PTP-mediated VE-cadherin phosphorylation and thus plays a protective role in ventilator-induced lung injury.
Keywords: Yes-associated protein; inflammation; vascular endothelial protein tyrosine phosphatase; vascular endothelial-cadherin; ventilator-induced lung injury.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
-
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. doi: 10.1056/nejm200005043421801. - DOI - PubMed
-
- Futier E, Constantin J-M, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant J-Y, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S, IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 369: 428–437, 2013. doi: 10.1056/NEJMoa1301082. - DOI - PubMed
-
- Neto AS, Cardoso SO, Manetta JA, Pereira VG, Espósito DC, Pasqualucci MO, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 308: 1651–1659, 2012. doi: 10.1001/jama.2012.13730. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

