Upregulated UCA1 contributes to oxaliplatin resistance of hepatocellular carcinoma through inhibition of miR-138-5p and activation of AKT/mTOR signaling pathway
- PMID: 33565716
- PMCID: PMC7874507
- DOI: 10.1002/prp2.720
Upregulated UCA1 contributes to oxaliplatin resistance of hepatocellular carcinoma through inhibition of miR-138-5p and activation of AKT/mTOR signaling pathway
Abstract
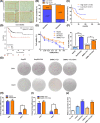
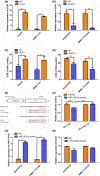
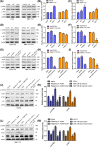
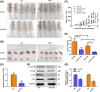
Hepatocellular carcinoma (HCC) inevitably developed oxaliplatin (OXA) resistance after long-term treatment, but the mechanism remains unclear. Here, we found that LncRNA UCA1 was upregulated in most of OXA-resistant HCC tissues and cells (HepG2/OXA and SMMC-7721/OXA). Follow-up analysis and online Kaplan-Meier Plotter revealed that HCC patients with high UCA1 level had a shorter survival compared with those with low expression. Overexpression of UCA1 increased OXA IC50 in HepG2 and SMMC-7721 cells, whereas knockdown of UCA1 decreased OXA IC50 in resistant counterparts. Moreover, dual luciferase reporter assay showed that co-transfection of UCA1-WT plasmid with miR-138-5p mimics enhanced fluorescence signals, whereas co-transfection of UCA1-Mut plasmid and miR-138-5p mimics did not induce any changes. Consistently, UCA1 levels in HepG2/OXA and SMMC-7721/OXA cells were downregulated after transfected with miR-138-5p mimics. UCA1 silencing or transfection of miR-138-5p mmics inhibited the activation of AKT and mTOR in HepG2/OXA and SMMC-7721/OXA cells, whereas UCA1 overexpression increased the phosphorylated AKT and mTOR levels in parental counterparts. Rapamycin or miR-138-5p mimics similarly suppressed the activation of AKT and mTOR, whereas UCA1 overexpression exert opposite roles. Interestingly, administration of rapamycin or miR-138-5p mimics apparently antagonized the effects of UCA1 on AKT and mTOR activation. Besides, depletion of UCA1 triggered more dramatic regression of HepG2 xenografts than that of HepG2/OXA xenografts with OXA treatment and impaired the p-AKT and p-mTOR levels in vivo. In conclusion, our findings provide the evidence that UCA1 may contribute to OXA resistance via miR-138-5p-mediated AK /mTOR activation, suggesting that UCA1 is a potential therapeutic target for HCC.
Keywords: UCA1; hepatocellular carcinoma; long noncoding RNA; miR-138-5p; oxaliplatin resistance.
© 2021 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
None.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Chang L, Hu Z, Zhou Z, Zhang H. Linc00518 contributes to multidrug resistance through regulating the MiR‐199a/MRP1 axis in breast cancer. Cell Physiol Biochem. 2018;48:16‐28. - PubMed
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. - PubMed
-
- Campagnolo L, Telesca C, Massimiani M, et al. Different expression of VEGF and EGFL7 in human hepatocellular carcinoma. Dig Liver Dis. 2016;48:76‐80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

