Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice
- PMID: 33566112
- PMCID: PMC7879582
- DOI: 10.1084/jem.20201803
Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice
Abstract
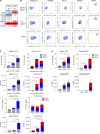
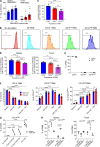
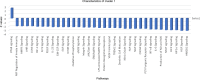
In this study, using single-cell RNA-seq, cell mass spectrometry, flow cytometry, and functional analysis, we characterized the heterogeneity of polymorphonuclear neutrophils (PMNs) in cancer. We describe three populations of PMNs in tumor-bearing mice: classical PMNs, polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), and activated PMN-MDSCs with potent immune suppressive activity. In spleens of mice, PMN-MDSCs gradually replaced PMNs during tumor progression. Activated PMN-MDSCs were found only in tumors, where they were present at the very early stages of the disease. These populations of PMNs in mice could be separated based on the expression of CD14. In peripheral blood of cancer patients, we identified two distinct populations of PMNs with characteristics of classical PMNs and PMN-MDSCs. The gene signature of tumor PMN-MDSCs was similar to that in mouse activated PMN-MDSCs and was closely associated with negative clinical outcome in cancer patients. Thus, we provide evidence that PMN-MDSCs are a distinct population of PMNs with unique features and potential for selective targeting opportunities.
© 2021 Veglia et al.
Conflict of interest statement
Disclosures: V. Bhargava is a full-time employee of Janssen Research and Development LLC. J. Patel and P. Wilkinson are employees of Janssen Research and Development LLC. D. Smirnov reported other from Janssen outside the submitted work and is an employee and shareholder of Janssen. M.A. Sepulveda is a full-time employee of Johnson & Johnson pharmaceuticals in Discovery Oncology. They collaborated with Dr. Gabrilovich and his team in the work included in this publication. R. Cristescu reported other from Merck during the conduct of the study and other from Merck outside the submitted work. A. Loboda is a full-time employee of Merck & Co. D.I. Gabrilovich reported other from AstraZeneca outside the submitted work and is a current employee of AstraZeneca. No other disclosures were reported.
Figures











References
-
- Al-Khami, A.A., Zheng L., Del Valle L., Hossain F., Wyczechowska D., Zabaleta J., Sanchez M.D., Dean M.J., Rodriguez P.C., and Ochoa A.C.. 2017. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. OncoImmunology. 6:e1344804. 10.1080/2162402X.2017.1344804 - DOI - PMC - PubMed
-
- Alshetaiwi, H., Pervolarakis N., McIntyre L.L., Ma D., Nguyen Q., Rath J.A., Nee K., Hernandez G., Evans K., Torosian L., et al. . 2020. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 5:eaay6017. 10.1126/sciimmunol.aay6017 - DOI - PMC - PubMed
-
- Amir, E.D., Davis K.L., Tadmor M.D., Simonds E.F., Levine J.H., Bendall S.C., Shenfeld D.K., Krishnaswamy S., Nolan G.P., and Pe’er D.. 2013. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 31(6):545–552. 10.1038/nbt.2594 - DOI - PMC - PubMed
-
- Ayers, M., Nebozhyn M., Cristescu R., McClanahan T.K., Perini R., Rubin E., Cheng J.D., Kaufman D.R., and Loboda A.. 2018. Molecular Profiling of Cohorts of Tumor Samples to Guide Clinical Development of Pembrolizumab as Monotherapy. Clin. Cancer Res. 25(5):1564–1573. 10.1158/1078-0432.CCR-18-1316 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

