Use of Biomarkers to Identify Acute Kidney Injury to Help Detect Sepsis in Patients With Infection
- PMID: 33566467
- PMCID: PMC7963439
- DOI: 10.1097/CCM.0000000000004845
Use of Biomarkers to Identify Acute Kidney Injury to Help Detect Sepsis in Patients With Infection
Abstract
Objectives: Although early recognition of sepsis is vital to improving outcomes, the diagnosis may be missed or delayed in many patients. Acute kidney injury is one of the most common organ failures in patients with sepsis but may not be apparent on presentation. Novel biomarkers for acute kidney injury might improve organ failure recognition and facilitate earlier sepsis care.
Design: Retrospective, international, Sapphire study.
Setting: Academic Medical Center.
Patients: Adults admitted to the ICU without evidence of acute kidney injury at time of enrollment.
Interventions: None.
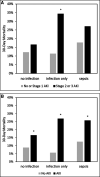
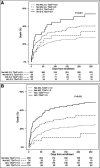
Measurements and main results: We stratified patients enrolled in the Sapphire study into three groups-those with a clinical diagnosis of sepsis (n = 216), those with infection without sepsis (n = 120), and those without infection (n = 387) at enrollment. We then examined 30-day mortality stratified by acute kidney injury within each group. Finally, we determined the operating characteristics for kidney stress markers (tissue inhibitor of metalloproteinases-2) × (insulin-like growth factor binding protein 7) for prediction of acute kidney injury as a sepsis-defining organ failure in patients with infection without a clinical diagnosis of sepsis at enrollment. Combining all groups, 30-day mortality was 23% for patients who developed stage 2-3 acute kidney injury within the first 3 days compared with 14% without stage 2-3 acute kidney injury. However, this difference was greatest in the infection without sepsis group (34% vs 11%; odds ratio, 4.09; 95% CI, 1.53-11.12; p = 0.005). Using a (tissue inhibitor of metalloproteinases-2) × (insulin-like growth factor binding protein 7) cutoff of 2.0 units, 14 patients (11.7%), in the infection/no sepsis group, tested positive of which 10 (71.4%) developed stage 2-3 acute kidney injury. The positive test result occurred a median of 19 hours (interquartile range, 0.8-34.0 hr) before acute kidney injury manifested by serum creatinine or urine output. Similar results were obtained using a cutoff of 1.0 for any stage of acute kidney injury.
Conclusions: Use of the urinary (tissue inhibitor of metalloproteinases-2) × (insulin-like growth factor binding protein 7) test could identify acute kidney injury in patients with infection, possibly helping to detect sepsis, nearly a day before acute kidney injury is apparent by clinical criteria.
Trial registration: ClinicalTrials.gov NCT01209169.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Dr. Kellum’s institution received funding from Astute Medical and bioMerieux. Drs. Kellum, Rimmelé, Shi, and Chawla received funding from Astute Medical. Drs. Kampf, Kwan, and McPherson are employees of Astute Medical/bioMerieux; Drs. Kellum, Rimmelé, Shi, and Chawla are paid consultants of Astute Medical/bioMerieux; and Dr. Kellum has received grant support from Astute Medical/bioMerieux apart from the current work. Dr. Artigas’s institution received funding from Lilly Foundation, and he received funding from Grifols, Fisher & Paykel. Drs. Kampf, Kwan, and McPherson received funding from Astute Medical (employee). Drs. Nguyen’s and Shapiro’s institutions received funding from Astute Medical. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- Vincent JL, Sakr Y, Sprung CL, et al. . Sepsis Occurrence in Acutely Ill Patients Investigators Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006; 34:344–353 - PubMed
-
- Bihorac A, Chawla LS, Shaw AD, et al. . Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014; 189:932–939 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

