Efference copy in kinesthetic perception: a copy of what is it?
- PMID: 33566734
- PMCID: PMC8282223
- DOI: 10.1152/jn.00545.2020
Efference copy in kinesthetic perception: a copy of what is it?
Abstract
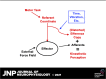
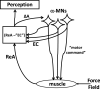
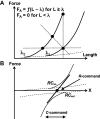
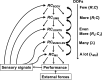
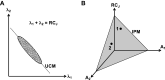
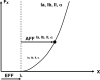
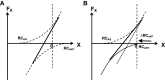
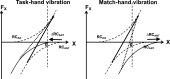
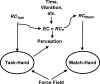
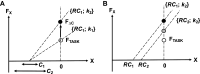
A number of notions in the fields of motor control and kinesthetic perception have been used without clear definitions. In this review, we consider definitions for efference copy, percept, and sense of effort based on recent studies within the physical approach, which assumes that the neural control of movement is based on principles of parametric control and involves defining time-varying profiles of spatial referent coordinates for the effectors. The apparent redundancy in both motor and perceptual processes is reconsidered based on the principle of abundance. Abundance of efferent and afferent signals is viewed as the means of stabilizing both salient action characteristics and salient percepts formalized as stable manifolds in high-dimensional spaces of relevant elemental variables. This theoretical scheme has led recently to a number of novel predictions and findings. These include, in particular, lower accuracy in perception of variables produced by elements involved in a multielement task compared with the same elements in single-element tasks, dissociation between motor and perceptual effects of muscle coactivation, force illusions induced by muscle vibration, and errors in perception of unintentional drifts in performance. Taken together, these results suggest that participation of efferent signals in perception frequently involves distorted copies of actual neural commands, particularly those to antagonist muscles. Sense of effort is associated with such distorted efferent signals. Distortions in efference copy happen spontaneously and can also be caused by changes in sensory signals, e.g., those produced by muscle vibration.
Keywords: coactivation; force matching; kinesthetic perception; referent coordinate; sense of effort.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author.
Figures
References
-
- Von Holst E, Mittelstaedt H. The reafference principle. In: The Behavioral Physiology of Animals and Man. The Collected Papers of Erich von Holst, translated by Martin R.Coral Gables, FL: University of Miami Press, pp. 139–173, 1950/1973.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

