Evidence of intermediate reticular formation involvement in swallow pattern generation, recorded optically in the neonate rat sagittally sectioned hindbrain
- PMID: 33566745
- PMCID: PMC8282227
- DOI: 10.1152/jn.00623.2020
Evidence of intermediate reticular formation involvement in swallow pattern generation, recorded optically in the neonate rat sagittally sectioned hindbrain
Abstract
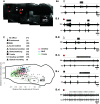
Swallow is a primitive behavior regulated by medullary networks, responsible for movement of food/liquid from the oral cavity to the esophagus. To investigate how functionally heterogeneous networks along the medullary intermediate reticular formation (IRt) and ventral respiratory column (VRC) control swallow, we electrically stimulated the nucleus tractus solitarius to induce fictive swallow between inspiratory bursts, with concurrent optical recordings using a synthetic Ca2+ indicator in the neonatal sagittally sectioned rat hindbrain (SSRH) preparation. Simultaneous recordings from hypoglossal nerve rootlet (XIIn) and ventral cervical spinal root C1-C2 enabled identification of the system-level correlates of 1) swallow (identified as activation of the XIIn but not the cervical root) and 2) Breuer-Hering expiratory reflex (BHE; lengthened expiration in response to stimuli during expiration). Optical recording revealed reconfiguration of respiration-modulated networks in the ventrolateral medulla during swallow and the BHE reflex. Recordings identified novel spatially compact networks in the IRt near the facial nucleus (VIIn) that were active during fictive swallow, suggesting that the swallow network is not restricted to the caudal medulla. These findings also establish the utility of using this in vitro preparation to investigate how functionally heterogeneous medullary networks interact and reconfigure to enable a repertoire of orofacial behaviors.NEW & NOTEWORTHY For the first time, medullary networks that control breathing and swallow are recorded optically. Episodic swallows are induced via electrical stimulation along the dorsal medulla, in and near the NTS, during spontaneously occurring fictive respiration. These findings establish that networks regulating both orofacial behaviors and breathing are accessible for optical recording at the surface of the sagittally sectioned rodent hindbrain preparation.
Keywords: Ca2+ imaging; medulla; rat; respiration; swallow.
Conflict of interest statement
This work was supported by National Heart, Lung, and Blood Institute Grant HL111215 (T. Pitts) and National Institute of Neurological Disorders and Stroke Grant NS110169 (T. Pitts).
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
Neurons in the dorsomedial medulla contribute to swallow pattern generation: Evidence of inspiratory activity during swallow.PLoS One. 2018 Jul 19;13(7):e0199903. doi: 10.1371/journal.pone.0199903. eCollection 2018. PLoS One. 2018. PMID: 30024913 Free PMC article.
-
Neurons in the Intermediate Reticular Nucleus Coordinate Postinspiratory Activity, Swallowing, and Respiratory-Sympathetic Coupling in the Rat.J Neurosci. 2019 Dec 4;39(49):9757-9766. doi: 10.1523/JNEUROSCI.0502-19.2019. Epub 2019 Oct 30. J Neurosci. 2019. PMID: 31666354 Free PMC article.
-
Swallowing-like activity elicited in neonatal rat medullary slice preparation.Brain Res. 2024 Aug 15;1837:148955. doi: 10.1016/j.brainres.2024.148955. Epub 2024 Apr 26. Brain Res. 2024. PMID: 38679314
-
The generation of pharyngeal phase of swallow and its coordination with breathing: interaction between the swallow and respiratory central pattern generators.Prog Brain Res. 2014;212:253-75. doi: 10.1016/B978-0-444-63488-7.00013-6. Prog Brain Res. 2014. PMID: 25194202 Review.
-
Role of the dorsal medulla in the neurogenesis of airway protection.Pulm Pharmacol Ther. 2015 Dec;35:105-10. doi: 10.1016/j.pupt.2015.10.012. Epub 2015 Nov 5. Pulm Pharmacol Ther. 2015. PMID: 26549786 Free PMC article. Review.
Cited by
-
Advances in Swallowing Neurophysiology across Pediatric Development: Current Evidence and Insights.Curr Phys Med Rehabil Rep. 2021 Dec;9(4):267-276. doi: 10.1007/s40141-021-00334-3. Epub 2021 Nov 18. Curr Phys Med Rehabil Rep. 2021. PMID: 34956736 Free PMC article.
-
Role of the postinspiratory complex in regulating swallow-breathing coordination and other laryngeal behaviors.Elife. 2023 Jun 5;12:e86103. doi: 10.7554/eLife.86103. Elife. 2023. PMID: 37272425 Free PMC article.
-
The Potential Role of Oxidative Stress in Modulating Airway Defensive Reflexes.Antioxidants (Basel). 2025 May 9;14(5):568. doi: 10.3390/antiox14050568. Antioxidants (Basel). 2025. PMID: 40427451 Free PMC article. Review.
-
Dendritic alterations precede age-related dysphagia and nucleus ambiguus motor neuron death.J Physiol. 2025 Mar;603(5):1299-1321. doi: 10.1113/JP287457. Epub 2025 Jan 27. J Physiol. 2025. PMID: 39868939 Free PMC article.
-
Roles for cerebellum and subsumption architecture in central pattern generation.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Mar;210(2):315-324. doi: 10.1007/s00359-023-01634-w. Epub 2023 May 2. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37130955 Free PMC article. Review.
References
-
- Dullemeijer P. The functional morphology of the head of the common viper. Arch Néerl Zool 11: 387–497, 1956. doi:10.1163/036551656X00139. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

