PREVALENCE OF NEUTRALIZING ANTIBODIES AGAINST POLIOVIRUS 1, 2, AND 3 IN HEALTHCARE PROFESSIONALS AGED 20-50 YEARS
- PMID: 33566984
- PMCID: PMC7870095
- DOI: 10.1590/1984-0462/2021/39/2019354
PREVALENCE OF NEUTRALIZING ANTIBODIES AGAINST POLIOVIRUS 1, 2, AND 3 IN HEALTHCARE PROFESSIONALS AGED 20-50 YEARS
Abstract
Objective: To describe the prevalence of neutralizing antibodies against poliovirus (PV1, PV2, and PV3) in blood samples of healthcare professionals aged 20 to 50 years.
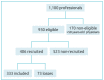
Methods: Health professionals who serve children at Darcy Vargas Children's Hospital and the Department of Pediatrics of Irmandade da Santa Casa de São Paulo. The sample size was calculated at 323 participants. The Mantel-Haenszel chi-square was used to verify differences between groups. The neutralization reaction detected human poliovirus antibodies. For susceptible individuals, vaccination with the inactivated+triple acellular polio vaccine was performed, and neutralizing antibodies were re-dosed after one week.
Results: 333 professionals were studied - 92.8% were immune to poliovirus 1, 86.5% to poliovirus 2, and 63.3% to poliovirus 3; 37% had titers less than 1:8 for any serotype, 5;1% had titers below 1:8 for all three. Vaccination with inactivated polio vaccine was performed for susceptible participants, and neutralizing antibodies were dosed after one week, showing increased titers for all polioviruses.
Conclusions: Despite the detection of a significant percentage of individuals with low poliovirus antibody titer, the challenge with vaccination demonstrated immune response compatible with poliovirus immunity.
Objetivo:: Descrever a prevalência de anticorpos neutralizantes contra poliovírus (tipos 1, 2 e 3) em amostra de sangue de profissionais de saúde com idade de 20 a 50 anos.
Métodos:: Profissionais de saúde que atendem crianças do Hospital Infantil Darcy Vargas e do Departamento de Pediatria da Irmandade da Santa Casa de São Paulo. O tamanho da amostra foi de 323 participantes. Os anticorpos contra poliovírus humanos foram detectados pela reação de neutralização. Para os indivíduos suscetíveis, foram administradas vacina para poliomielite inativada+tríplice e nova dosagem de anticorpos neutralizantes após uma semana. Utilizou-se o teste do qui-quadrado de Mantel-Haenszel para verificar as diferenças entre os grupos.
Resultados:: Foram estudados 333 profissionais - 92,8% eram imunes ao poliovírus 1; 86,5%, ao poliovírus 2; 63,57%, ao poliovírus 3; 37% apresentaram títulos inferiores a 1:8 para qualquer sorotipo; 5,1% tinham títulos abaixo de 1:8 para os três. Após a vacinação dos suscetíveis, houve elevação dos títulos para todos os poliovírus.
Conclusões:: Apesar da detecção de percentual significativo de indivíduos com baixo título de anticorpos para poliovírus, o desafio da vacinação demonstrou resposta imune robusta compatível.
Conflict of interest statement
Figures
References
-
- World Health Organization . Polio this week as of 08 January 2020. Geneva: WHO; 2020. [2020 Jan 08]. [homepage on the Internet] Available from: http://polioeradication.org/polio-today/polio-now/this-week/
-
- Prevots DR, Ciofi degli Atti ML, Sallabanda A, Diamante E, Aylward RB, Kakariqqi E, et al. Outbreak of paralytic poliomyelitis in Albania, 1996: high attack rate among adults and apparent interruption of transmission following nationwide mass vaccination. Clin Infect Dis. 1998;26:419–425. doi: 10.1086/516312. - DOI - PubMed
-
- Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine - live. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Philadelphia: Saunders Elsevier; 2008. pp. 605–630.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical