Mechanism of gating and partial agonist action in the glycine receptor
- PMID: 33567265
- PMCID: PMC8115384
- DOI: 10.1016/j.cell.2021.01.026
Mechanism of gating and partial agonist action in the glycine receptor
Abstract
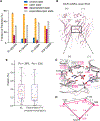
Ligand-gated ion channels mediate signal transduction at chemical synapses and transition between resting, open, and desensitized states in response to neurotransmitter binding. Neurotransmitters that produce maximum open channel probabilities (Po) are full agonists, whereas those that yield lower than maximum Po are partial agonists. Cys-loop receptors are an important class of neurotransmitter receptors, yet a structure-based understanding of the mechanism of partial agonist action has proven elusive. Here, we study the glycine receptor with the full agonist glycine and the partial agonists taurine and γ-amino butyric acid (GABA). We use electrophysiology to show how partial agonists populate agonist-bound, closed channel states and cryo-EM reconstructions to illuminate the structures of intermediate, pre-open states, providing insights into previously unseen conformational states along the receptor reaction pathway. We further correlate agonist-induced conformational changes to Po across members of the receptor family, providing a hypothetical mechanism for partial and full agonist action at Cys-loop receptors.
Keywords: SMA; cryo-EM; gating mechanism; glycine receptor; ligand-gated ion channels; partial agonists action.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





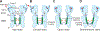
Comment in
-
Partial agonists go molecular.Trends Pharmacol Sci. 2021 Jul;42(7):507-509. doi: 10.1016/j.tips.2021.04.008. Epub 2021 May 6. Trends Pharmacol Sci. 2021. PMID: 33965248
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

