Quantification and Optimization of Standard-of-Care Therapy to Delay the Emergence of Resistant Bone Metastatic Prostate Cancer
- PMID: 33567529
- PMCID: PMC7915310
- DOI: 10.3390/cancers13040677
Quantification and Optimization of Standard-of-Care Therapy to Delay the Emergence of Resistant Bone Metastatic Prostate Cancer
Abstract
Background: Bone metastatic prostate cancer (BMPCa), despite the initial responsiveness to androgen deprivation therapy (ADT), inevitably becomes resistant. Recent clinical trials with upfront treatment of ADT combined with chemotherapy or novel hormonal therapies (NHTs) have extended overall patient survival. These results indicate that there is significant potential for the optimization of standard-of-care therapies to delay the emergence of progressive metastatic disease.
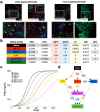
Methods: Here, we used data extracted from human bone metastatic biopsies pre- and post-abiraterone acetate/prednisone to generate a mathematical model of bone metastatic prostate cancer that can unravel the treatment impact on disease progression. Intra-tumor heterogeneity in regard to ADT and chemotherapy resistance was derived from biopsy data at a cellular level, permitting the model to track the dynamics of resistant phenotypes in response to treatment from biological first-principles without relying on data fitting. These cellular data were mathematically correlated with a clinical proxy for tumor burden, utilizing prostate-specific antigen (PSA) production as an example.
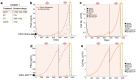
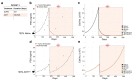
Results: Using this correlation, our model recapitulated the individual patient response to applied treatments in a separate and independent cohort of patients (n = 24), and was able to estimate the initial resistance to the ADT of each patient. Combined with an intervention-decision algorithm informed by patient-specific prediction of initial resistance, we propose to optimize the sequence of treatments for each patient with the goal of delaying the evolution of resistant disease and limit cancer cell growth, offering evidence for an improvement against retrospective data.
Conclusions: Our results show how minimal but widely available patient information can be used to model and track the progression of BMPCa in real time, offering a clinically relevant insight into the patient-specific evolutionary dynamics of the disease and suggesting new therapeutic options for intervention.
Trial registration: NCT # 01953640.
Funding: Funded by an NCI U01 (NCI) U01CA202958-01 and a Moffitt Team Science Award. CCL and DB were partly funded by an NCI PSON U01 (U01CA244101). AA was partly funded by a Department of Defense Prostate Cancer Research Program (W81XWH-15-1-0184) fellowship. LC was partly funded by a postdoctoral fellowship (PF-13-175-01-CSM) from the American Cancer Society.
Keywords: androgen therapy resistance; bone metastatic prostate cancer; computational model; mathematical oncology; personalized treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Morrissey C., Roudier M.P., Dowell A., True L.D., Ketchanji M., Welty C., Corey E., Lange P.H., Higano C.S., Vessella R.L. Effects of androgen deprivation therapy and bisphosphonate treatment on bone in patients with metastatic castration-resistant prostate cancer: Results from the University of Washington Rapid Autopsy Series. J. Bone Miner. Res. 2013;28:333–340. doi: 10.1002/jbmr.1749. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

