Genomic Landscape of Hodgkin Lymphoma
- PMID: 33567641
- PMCID: PMC7915917
- DOI: 10.3390/cancers13040682
Genomic Landscape of Hodgkin Lymphoma
Abstract
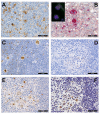
Background: Hodgkin lymphoma (HL) is predominantly composed of reactive, non-neoplastic cells surrounding scarcely distributed tumor cells, that is, so-called Hodgkin and Reed-Sternberg (HRS) or lymphocyte predominant (LP) cells. This scarcity impeded the analysis of the tumor cell genomes for a long time, but recently developed methods (especially laser capture microdissection, flow cytometry/fluorescence-activated cell sorting) facilitated molecular investigation, elucidating the pathophysiological principles of "Hodgkin lymphomagenesis".
Methods: We reviewed the relevant literature of the last three decades focusing on the genomic landscape of classic and nodular lymphocyte predominant HL (NLPHL) and summarized molecular cornerstones.
Results: Firstly, the malignant cells of HL evade the immune system by altered expression of PDL1/2, B2M and MHC class I and II due to various genetic alterations. Secondly, tumor growth is promoted by permanently activated JAK/STAT signaling due to pervasive mutations of multiple genes involved in the pathway. Thirdly, apoptosis of neoplastic cells is prevented by alterations of NF-κB compounds and the PI3K/AKT/mTOR axis. Additionally, Epstein-Barr virus infection can simultaneously activate JAK/STAT and NF-κB, similarly leading to enhanced survival and evasion of apoptosis. Finally, epigenetic phenomena such as promoter hypermethylation lead to the downregulation of B-lineage-specific, tumor-suppressor and immune regulation genes.
Conclusion: The blueprint of HL genomics has been laid, paving the way for future investigations into its complex pathophysiology.
Keywords: 9p24 locus; Epstein-Barr virus; Hodgkin lymphoma; JAK/STAT; NF-κB; PDL1; PDL2; PI3K/AKT/mTOR; cell enrichment; cell lines; epigenetics; genomes; mutations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Atayar C., Kok K., Kluiver J., Bosga A., van den Berg E., van der Vlies P., Blokzijl T., Harms G., Davelaar I., Sikkema-Raddatz B., et al. BCL6 alternative breakpoint region break and homozygous deletion of 17q24 in the nodular lymphocyte predominance type of Hodgkin’s lymphoma-derived cell line DEV. Hum. Pathol. 2006;37:675–683. doi: 10.1016/j.humpath.2006.01.018. - DOI - PubMed
-
- Hartmann S., Martin-Subero J.I., Gesk S., Hüsken J., Giefing M., Nagel I., Riemke J., Chott A., Klapper W., Parrens M., et al. Detection of genomic imbalances in microdissected Hodgkin and Reed-Sternberg cells of classical Hodgkin’s lymphoma by array-based comparative genomic hybridization. Haematologica. 2008;93:1318–1326. doi: 10.3324/haematol.12875. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

