"Cell Membrane Theory of Senescence" and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications
- PMID: 33567774
- PMCID: PMC7914625
- DOI: 10.3390/biom11020241
"Cell Membrane Theory of Senescence" and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications
Abstract
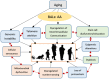
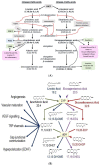
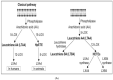
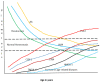
Lipids are an essential constituent of the cell membrane of which polyunsaturated fatty acids (PUFAs) are the most important component. Activation of phospholipase A2 (PLA2) induces the release of PUFAs from the cell membrane that form precursors to both pro- and ant-inflammatory bioactive lipids that participate in several cellular processes. PUFAs GLA (gamma-linolenic acid), DGLA (dihomo-GLA), AA (arachidonic acid), EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are derived from dietary linoleic acid (LA) and alpha-linolenic acid (ALA) by the action of desaturases whose activity declines with age. Consequently, aged cells are deficient in GLA, DGLA, AA, AA, EPA and DHA and their metabolites. LA, ALA, AA, EPA and DHA can also be obtained direct from diet and their deficiency (fatty acids) may indicate malnutrition and deficiency of several minerals, trace elements and vitamins some of which are also much needed co-factors for the normal activity of desaturases. In many instances (patients) the plasma and tissue levels of GLA, DGLA, AA, EPA and DHA are low (as seen in patients with hypertension, type 2 diabetes mellitus) but they do not have deficiency of other nutrients. Hence, it is reasonable to consider that the deficiency of GLA, DGLA, AA, EPA and DHA noted in these conditions are due to the decreased activity of desaturases and elongases. PUFAs stimulate SIRT1 through protein kinase A-dependent activation of SIRT1-PGC1α complex and thus, increase rates of fatty acid oxidation and prevent lipid dysregulation associated with aging. SIRT1 activation prevents aging. Of all the SIRTs, SIRT6 is critical for intermediary metabolism and genomic stability. SIRT6-deficient mice show shortened lifespan, defects in DNA repair and have a high incidence of cancer due to oncogene activation. SIRT6 overexpression lowers LDL and triglyceride level, improves glucose tolerance, and increases lifespan of mice in addition to its anti-inflammatory effects at the transcriptional level. PUFAs and their anti-inflammatory metabolites influence the activity of SIRT6 and other SIRTs and thus, bring about their actions on metabolism, inflammation, and genome maintenance. GLA, DGLA, AA, EPA and DHA and prostaglandin E2 (PGE2), lipoxin A4 (LXA4) (pro- and anti-inflammatory metabolites of AA respectively) activate/suppress various SIRTs (SIRt1 SIRT2, SIRT3, SIRT4, SIRT5, SIRT6), PPAR-γ, PARP, p53, SREBP1, intracellular cAMP content, PKA activity and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1-α). This implies that changes in the metabolism of bioactive lipids as a result of altered activities of desaturases, COX-2 and 5-, 12-, 15-LOX (cyclo-oxygenase and lipoxygenases respectively) may have a critical role in determining cell age and development of several aging associated diseases and genomic stability and gene and oncogene activation. Thus, methods designed to maintain homeostasis of bioactive lipids (GLA, DGLA, AA, EPA, DHA, PGE2, LXA4) may arrest aging process and associated metabolic abnormalities.
Keywords: aging; bioactive lipids; cell membrane; inflammation; sirtuins; unsaturated fatty acids.
Conflict of interest statement
Author UND is employed by the company UND Life Sciences.
Figures







References
-
- Carmona J.J., Michan S. Biology of Healthy Aging and Longevity. Rev. Invest. Clin. 2016;68:7–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

