Methotrexate-related central neurotoxicity: clinical characteristics, risk factors and genome-wide association study in children treated for acute lymphoblastic leukemia
- PMID: 33567813
- PMCID: PMC8883571
- DOI: 10.3324/haematol.2020.268565
Methotrexate-related central neurotoxicity: clinical characteristics, risk factors and genome-wide association study in children treated for acute lymphoblastic leukemia
Abstract
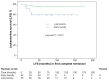
Symptomatic methotrexate-related central neurotoxicity (MTX neurotoxicity) is a severe toxicity experienced during acute lymphoblastic leukemia (ALL) therapy with potential long-term neurologic complications. Risk factors and long-term outcomes require further study. We conducted a systematic, retrospective review of 1,251 consecutive Australian children enrolled on Berlin-Frankfurt-Münster or Children's Oncology Group-based protocols between 1998-2013. Clinical risk predictors for MTX neurotoxicity were analyzed using regression. A genome-wide association study (GWAS) was performed on 48 cases and 537 controls. The incidence of MTX neurotoxicity was 7.6% (n=95 of 1,251), at a median of 4 months from ALL diagnosis and 8 days after intravenous or intrathecal MTX. Grade 3 elevation of serum aspartate aminotransferase (P=0.005, odds ratio 2.31 [range, 1.28-4.16]) in induction/consolidation was associated with MTX neurotoxicity, after accounting for the only established risk factor, age ≥10 years. Cumulative incidence of CNS relapse was increased in children where intrathecal MTX was omitted following symptomatic MTX neurotoxicity (n=48) compared to where intrathecal MTX was continued throughout therapy (n=1,174) (P=0.047). Five-year central nervous system relapse-free survival was 89.2 4.6% when intrathecal MTX was ceased compared to 95.4 0.6% when intrathecal MTX was continued. Recurrence of MTX neurotoxicity was low (12.9%) for patients whose intrathecal MTX was continued after their first episode. The GWAS identified single-nucletide polymorphism associated with MTX neurotoxicity near genes regulating neuronal growth, neuronal differentiation and cytoskeletal organization (P<1x10-6). In conclusion, increased serum aspartate aminotransferase and age ≥10 years at diagnosis were independent risk factors for MTX neurotoxicity. Our data do not support cessation of intrathecal MTX after a first MTX neurotoxicity event.
Figures

References
-
- Badke C, Fleming A, Iqbal A, et al. . Rechallenging with intrathecal methotrexate after developing subacute neurotoxicity in children with hematologic malignancies. Pediatr Blood Cancer. 2016;63(4):723-726. - PubMed
-
- Rubnitz J, Relling M, Harrison P, et al. . Transient encephalopathy following highdose methotrexate treatment in childhood acute lymphoblastic leukemia. Leukemia. 1998;12(8):1176-1181. - PubMed
-
- Schmiegelow K, Attarbaschi A, Barzilai S, et al. . Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17(6):e231-e239. - PubMed
-
- Bond J, Hough R, Moppett J, Vora A, Mitchell C, Goulden N. ‘Stroke-like syndrome’caused by intrathecal methotrexate in patients treated during the UKALL 2003 trial. Leukemia. 2013;27(4):954-956. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

