Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade
- PMID: 33568350
- PMCID: PMC7878154
- DOI: 10.1136/jitc-2020-001884
Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade
Abstract
Background: Pneumonitis related to immune checkpoint blockade is uncommon but can be severe, fatal or chronic. Steroids are first-line treatment, however, some patients are refractory or become resistant to steroids. Like many immune-related adverse events, little is known regarding the outcomes and optimal management of patients in whom steroids are ineffective.
Methods: We performed a single-center retrospective cohort study at a high-volume tertiary cancer center to evaluate the clinical course, management strategies and outcomes of patients treated for immune checkpoint pneumonitis with immune modulatory medications in addition to systemic steroids. Pharmacy records were queried for patients treated with both immune checkpoint blockade and receipt of additional immune modulators. Records were then manually reviewed to identify patients who received the additional immune modulators for immune checkpoint pneumonitis.
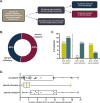
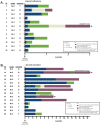
Results: From 2013 to 2020, we identified 26 patients treated for immune checkpoint pneumonitis with additional immune modulators in addition to steroids. Twelve patients (46%) were steroid-refractory and 14 (54%) were steroid-resistant. Pneumonitis severity included grade 2 (42%) or grade 3-4 (58%). Additional immune modulation consisted of tumor necrosis factor-alpha inhibitor (77%) and/or mycophenolate (23%). Durable improvement in pneumonitis following initiation of additional immune modulators occurred in 10 patients (38%), including three patients (12%) in whom pneumonitis resolved and all immunosuppressants ceased. The rate of 90-day all-cause mortality/hospice referral was 50%. At last follow-up, mortality attributable to pneumonitis was 23%. In addition to mortality from pneumonitis and cancer, 3 patients (12%) died due to infections possibly associated with immunosuppression.
Conclusions: Steroid-refractory or -resistant immune checkpoint pneumonitis is uncommon but associated with significant morbidity and mortality. Additional immunomodulators can yield durable improvement, attained in over one third of patients. An improved understanding of the underlying biology of immune-related pneumonitis will be crucial to guide more precise and effective treatment strategies in the future.
Trial registration: ClinicalTrials.gov NCT04438382.
Keywords: immunotherapy; inflammation.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JB, HR, PF, AS, I-HL, NJS, MC report no COI. JL reports honoraria from Targeted Oncology. MP reports consulting fees from BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro, Eisai; honoraria from BMS and Merck; institutional support from RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, AstraZeneca. MC reports institutional research support and employment of a family member by Bristol-Myers Squibb; consulting, advisory, or speaking compensation for: AstraZeneca/MedImmune, Incyte, Moderna, Immunocore and Merck. MHV reports receiving commercial research grants from Bristol-Myers Squibb, Pfizer; honoraria from BMS, travel/accommodation from Astra Zeneca, Eisai; consultant/advisory board member for Corvus Pharmaceuticals, Exelixis, Eisai, Merck, Onquality Pharmaceuticals and Pfizer as well as institutional support from Astra Zeneca, BMS, Corvus, Medimmune, Merck, Pfizer. ABW reports consulting fees from Nanobiotix; honorarium from LG Chem Life Sciences, Novartis; advisory board member of Lovance. MDH reports research support from Bristol-Myers Squibb; consulting fees from Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Shattuck Labs, Immunai, Blueprint Medicines, Achilles and Arcus; travel support/honoraria from AstraZeneca, Eli Lilly and Bristol-Myers Squibb; options from Shattuck Labs, Immunai and Arcus; patent filed by his institution related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx.
Figures

References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
