APEX2 Proximity Proteomics Resolves Flagellum Subdomains and Identifies Flagellum Tip-Specific Proteins in Trypanosoma brucei
- PMID: 33568455
- PMCID: PMC8141408
- DOI: 10.1128/mSphere.01090-20
APEX2 Proximity Proteomics Resolves Flagellum Subdomains and Identifies Flagellum Tip-Specific Proteins in Trypanosoma brucei
Abstract
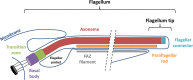
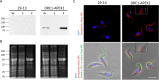
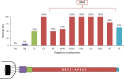
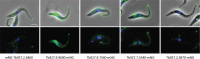
Trypanosoma brucei is the protozoan parasite responsible for sleeping sickness, a lethal vector-borne disease. T. brucei has a single flagellum (cilium) that plays critical roles in transmission and pathogenesis. An emerging concept is that the flagellum is organized into subdomains, each having specialized composition and function. The overall flagellum proteome has been well studied, but a critical knowledge gap is the protein composition of individual subdomains. We have tested whether APEX-based proximity proteomics could be used to examine the protein composition of T. brucei flagellum subdomains. As APEX-based labeling has not previously been described in T. brucei, we first fused APEX2 to the DRC1 subunit of the nexin-dynein regulatory complex, a well-characterized axonemal complex. We found that DRC1-APEX2 directs flagellum-specific biotinylation, and purification of biotinylated proteins yields a DRC1 "proximity proteome" having good overlap with published proteomes obtained from purified axonemes. Having validated the use of APEX2 in T. brucei, we next attempted to distinguish flagellar subdomains by fusing APEX2 to a flagellar membrane protein that is restricted to the flagellum tip, AC1, and another one that is excluded from the tip, FS179. Fluorescence microscopy demonstrated subdomain-specific biotinylation, and principal-component analysis showed distinct profiles between AC1-APEX2 and FS179-APEX2. Comparing these two profiles allowed us to identify an AC1 proximity proteome that is enriched for tip proteins, including proteins involved in signaling. Our results demonstrate that APEX2-based proximity proteomics is effective in T. brucei and can be used to resolve the proteome composition of flagellum subdomains that cannot themselves be readily purified.IMPORTANCE Sleeping sickness is a neglected tropical disease caused by the protozoan parasite Trypanosoma brucei The disease disrupts the sleep-wake cycle, leading to coma and death if left untreated. T. brucei motility, transmission, and virulence depend on its flagellum (cilium), which consists of several different specialized subdomains. Given the essential and multifunctional role of the T. brucei flagellum, there is need for approaches that enable proteomic analysis of individual subdomains. Our work establishes that APEX2 proximity labeling can, indeed, be implemented in the biochemical environment of T. brucei and has allowed identification of proximity proteomes for different flagellar subdomains that cannot be purified. This capacity opens the possibility to study the composition and function of other compartments. We expect this approach may be extended to other eukaryotic pathogens and will enhance the utility of T. brucei as a model organism to study ciliopathies, heritable human diseases in which cilium function is impaired.
Keywords: Trypanosoma; cell signaling; flagella.
Copyright © 2021 Vélez-Ramírez et al.
Figures



References
-
- FAO. 2019. Controling tsetse and trypanosomosis to protect African livestock keepers, public health and farmers' livelihoods. FAO, Rome, Italy.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

