Uncovering the Hidden Credentials of Brucella Virulence
- PMID: 33568459
- PMCID: PMC8549849
- DOI: 10.1128/MMBR.00021-19
Uncovering the Hidden Credentials of Brucella Virulence
Abstract
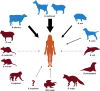
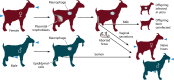
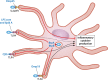
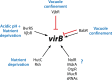
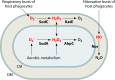
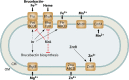
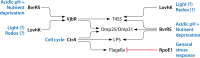
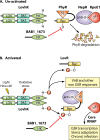
Bacteria in the genus Brucella are important human and veterinary pathogens. The abortion and infertility they cause in food animals produce economic hardships in areas where the disease has not been controlled, and human brucellosis is one of the world's most common zoonoses. Brucella strains have also been isolated from wildlife, but we know much less about the pathobiology and epidemiology of these infections than we do about brucellosis in domestic animals. The brucellae maintain predominantly an intracellular lifestyle in their mammalian hosts, and their ability to subvert the host immune response and survive and replicate in macrophages and placental trophoblasts underlies their success as pathogens. We are just beginning to understand how these bacteria evolved from a progenitor alphaproteobacterium with an environmental niche and diverged to become highly host-adapted and host-specific pathogens. Two important virulence determinants played critical roles in this evolution: (i) a type IV secretion system that secretes effector molecules into the host cell cytoplasm that direct the intracellular trafficking of the brucellae and modulate host immune responses and (ii) a lipopolysaccharide moiety which poorly stimulates host inflammatory responses. This review highlights what we presently know about how these and other virulence determinants contribute to Brucella pathogenesis. Gaining a better understanding of how the brucellae produce disease will provide us with information that can be used to design better strategies for preventing brucellosis in animals and for preventing and treating this disease in humans.
Keywords: Brucella; pathogenesis; virulence determinants.
Copyright © 2021 American Society for Microbiology.
Figures





References
-
- Soler-Lloréns PF, Quance CR, Lawhon SD, Stuber TP, Edwards JF, Ficht TA, Robbe-Austerman S, O’Callaghan D, Keriel A. 2016. A Brucella spp. isolate from a Pac-Man frog (Ceratophrys ornata) reveals characteristics departing from classical brucellae. Front Cell Infect Microbiol 6:116. doi:10.3389/fcimb.2016.00116. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

