Targeted immunotherapy for HER2-low breast cancer with 17p loss
- PMID: 33568521
- PMCID: PMC8351376
- DOI: 10.1126/scitranslmed.abc6894
Targeted immunotherapy for HER2-low breast cancer with 17p loss
Abstract
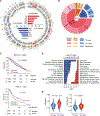
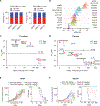
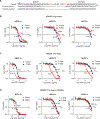
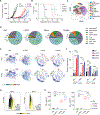
The clinical challenge for treating HER2 (human epidermal growth factor receptor 2)-low breast cancer is the paucity of actionable drug targets. HER2-targeted therapy often has poor clinical efficacy for this disease due to the low level of HER2 protein on the cancer cell surface. We analyzed breast cancer genomics in the search for potential drug targets. Heterozygous loss of chromosome 17p is one of the most frequent genomic events in breast cancer, and 17p loss involves a massive deletion of genes including the tumor suppressor TP53 Our analyses revealed that 17p loss leads to global gene expression changes and reduced tumor infiltration and cytotoxicity of T cells, resulting in immune evasion during breast tumor progression. The 17p deletion region also includes POLR2A, a gene encoding the catalytic subunit of RNA polymerase II that is essential for cell survival. Therefore, breast cancer cells with heterozygous loss of 17p are extremely sensitive to the inhibition of POLR2A via a specific small-molecule inhibitor, α-amanitin. Here, we demonstrate that α-amanitin-conjugated trastuzumab (T-Ama) potentiated the HER2-targeted therapy and exhibited superior efficacy in treating HER2-low breast cancer with 17p loss. Moreover, treatment with T-Ama induced immunogenic cell death in breast cancer cells and, thereby, delivered greater efficacy in combination with immune checkpoint blockade therapy in preclinical HER2-low breast cancer models. Collectively, 17p loss not only drives breast tumorigenesis but also confers therapeutic vulnerabilities that may be used to develop targeted precision immunotherapy.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


References
-
- Chen LC, Neubauer A, Kurisu W, Waldman FM, Ljung BM, Goodson III W, Goldman ES, Moore II D, Balazs M, Liu E., Loss of heterozygosity on the short arm of chromosome 17 is associated with high proliferative capacity and DNA aneuploidy in primary human breast cancer. Proc. Natl. Acad. Sci. U.S.A 88, 3847–3851 (1991). - PMC - PubMed
-
- Li Y, Liu Y, Xu H, Jiang G, Van der Jeught K, Fang Y, Zhou Z, Zhang L, Frieden M, Wang L, Luo Z, Radovich M, Schneider BP, Deng Y, Liu Y, Huang K, He B, Wang J, He X, Zhang X, Ji G, Lu X., Heterozygous deletion of chromosome 17p renders prostate cancer vulnerable to inhibition of RNA polymerase II. Nat. Commun 9, 4394 (2018). - PMC - PubMed
-
- Menendez D, Inga A, Resnick MA, The expanding universe of p53 targets. Nat. Rev. Cancer 9, 724–737 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

