Nicotinamide for the treatment of heart failure with preserved ejection fraction
- PMID: 33568522
- PMCID: PMC7611499
- DOI: 10.1126/scitranslmed.abd7064
Nicotinamide for the treatment of heart failure with preserved ejection fraction
Abstract
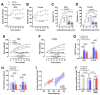
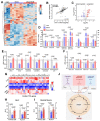
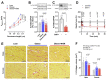
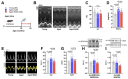
Heart failure with preserved ejection fraction (HFpEF) is a highly prevalent and intractable form of cardiac decompensation commonly associated with diastolic dysfunction. Here, we show that diastolic dysfunction in patients with HFpEF is associated with a cardiac deficit in nicotinamide adenine dinucleotide (NAD+). Elevating NAD+ by oral supplementation of its precursor, nicotinamide, improved diastolic dysfunction induced by aging (in 2-year-old C57BL/6J mice), hypertension (in Dahl salt-sensitive rats), or cardiometabolic syndrome (in ZSF1 obese rats). This effect was mediated partly through alleviated systemic comorbidities and enhanced myocardial bioenergetics. Simultaneously, nicotinamide directly improved cardiomyocyte passive stiffness and calcium-dependent active relaxation through increased deacetylation of titin and the sarcoplasmic reticulum calcium adenosine triphosphatase 2a, respectively. In a long-term human cohort study, high dietary intake of naturally occurring NAD+ precursors was associated with lower blood pressure and reduced risk of cardiac mortality. Collectively, these results suggest NAD+ precursors, and especially nicotinamide, as potential therapeutic agents to treat diastolic dysfunction and HFpEF in humans.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



Comment in
-
Restoration of NAD+ levels as a therapy for HFpEF.Nat Rev Cardiol. 2021 May;18(5):307. doi: 10.1038/s41569-021-00535-2. Nat Rev Cardiol. 2021. PMID: 33649584 No abstract available.
References
-
- Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. - PubMed
-
- Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. OPTIMIZE-HF Investigators and Hospitals, Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. - PubMed
-
- Lourenço AP, Leite-Moreira AF, Balligand J-L, Bauersachs J, Dawson D, de Boer RA, de Windt LJ, Falcão-Pires I, Fontes-Carvalho R, Franz S, Giacca M, et al. An integrative translational approach to study heart failure with preserved ejection fraction: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur J Heart Fail. 2018;20:216–227. - PubMed
-
- Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

