Understanding the Impact of Resistance to Influenza Antivirals
- PMID: 33568554
- PMCID: PMC7950363
- DOI: 10.1128/CMR.00224-20
Understanding the Impact of Resistance to Influenza Antivirals
Abstract
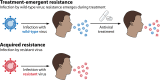
Influenza poses a significant burden on society and health care systems. Although antivirals are an integral tool in effective influenza management, the potential for the emergence of antiviral-resistant viruses can lead to uncertainty and hesitation among front-line prescribers and policy makers. Here, we provide an overview of influenza antiviral resistance in context, exploring the key concepts underlying its development and clinical impact. Due to the acute nature of influenza in immunocompetent patients, resistant viruses that develop during antiviral treatment of a single patient ("treatment-emergent resistance") are usually cleared in a relatively short time, with no impact on future antiviral efficacy. In addition, although available data are limited by small numbers of patients, they show that antiviral treatment still provides clinical benefit to the patient within whom resistance emerges. In contrast, the sustained community transmission of resistant variants in the absence of treatment ("acquired resistance") is of greater concern and can potentially render front-line antivirals ineffective. Importantly, however, resistant viruses are usually associated with reduced fitness such that their widespread transmission is relatively rare. Influenza antivirals are an essential part of effective influenza management due to their ability to reduce the risk of complications and death in infected patients. Although antiviral resistance should be taken seriously and requires continuous careful monitoring, it is not comparable to antibiotic resistance in bacteria, which can become permanent and widespread, with far-reaching medical consequences. The benefits of antiviral treatment far outweigh concerns of potential resistance, which in the vast majority of cases does not have a significant clinical impact.
Keywords: antiviral agents; drug resistance evolution; influenza.
Copyright © 2021 American Society for Microbiology.
Figures

References
-
- WHO. 2019. Global influenza strategy 2019-2030. WHO, Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/311184/9789241515320-en.... Accessed 19 January 2021.
-
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson M-A, Bresee JS, Global Seasonal Influenza-Associated Mortality Collaborator Network . 2018. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
-
- Johns Hopkins University. 2020. COVID-19 dashboard. Johns Hopkins University, Baltimore, MD. https://coronavirus.jhu.edu/map.html. Accessed 19 January 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

