Replication dynamics of recombination-dependent replication forks
- PMID: 33568651
- PMCID: PMC7876095
- DOI: 10.1038/s41467-021-21198-0
Replication dynamics of recombination-dependent replication forks
Abstract
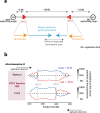
Replication forks restarted by homologous recombination are error prone and replicate both strands semi-conservatively using Pol δ. Here, we use polymerase usage sequencing to visualize in vivo replication dynamics of HR-restarted forks at an S. pombe replication barrier, RTS1, and model replication by Monte Carlo simulation. We show that HR-restarted forks synthesise both strands with Pol δ for up to 30 kb without maturing to a δ/ε configuration and that Pol α is not used significantly on either strand, suggesting the lagging strand template remains as a gap that is filled in by Pol δ later. We further demonstrate that HR-restarted forks progress uninterrupted through a fork barrier that arrests canonical forks. Finally, by manipulating lagging strand resection during HR-restart by deleting pku70, we show that the leading strand initiates replication at the same position, signifying the stability of the 3' single strand in the context of increased resection.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

