Pluripotency and immunomodulatory signatures of canine induced pluripotent stem cell-derived mesenchymal stromal cells are similar to harvested mesenchymal stromal cells
- PMID: 33568729
- PMCID: PMC7875972
- DOI: 10.1038/s41598-021-82856-3
Pluripotency and immunomodulatory signatures of canine induced pluripotent stem cell-derived mesenchymal stromal cells are similar to harvested mesenchymal stromal cells
Abstract
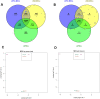
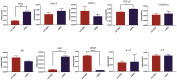
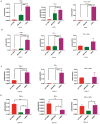
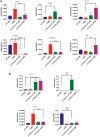
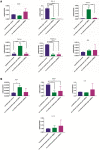
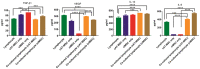
With a view towards harnessing the therapeutic potential of canine mesenchymal stromal cells (cMSCs) as modulators of inflammation and the immune response, and to avoid the issues of the variable quality and quantity of harvested cMSCs, we examined the immunomodulatory properties of cMSCs derived from canine induced pluripotent stem cells (ciMSCs), and compared them to cMSCs harvested from adipose tissue (cAT-MSC) and bone marrow (cBM-MSC). A combination of deep sequencing and quantitative RT-PCR of the ciMSC transcriptome confirmed that ciMSCs express more genes in common with cBM-MSCs and cAT-MSCs than with the ciPSCs from which they were derived. Both ciMSCs and harvested cMSCs express a range of pluripotency factors in common with the ciPSCs including NANOG, POU5F1 (OCT-4), SOX-2, KLF-4, LIN-28A, MYC, LIF, LIFR, and TERT. However, ESRRB and PRDM-14, both factors associated with naïve, rather than primed, pluripotency were expressed only in the ciPSCs. CXCR-4, which is essential for the homing of MSCs to sites of inflammation, is also detectable in ciMSCs, cAT- and cBM-MSCs, but not ciPSCs. ciMSCs constitutively express the immunomodulatory factors iNOS, GAL-9, TGF-β1, PTGER-2α and VEGF, and the pro-inflammatory mediators COX-2, IL-1β and IL-8. When stimulated with the canine pro-inflammatory cytokines tumor necrosis factor-α (cTNF-α), interferon-γ (cIFN-γ), or a combination of both, ciMSCs upregulated their expression of IDO, iNOS, GAL-9, HGF, TGF-β1, PTGER-2α, VEGF, COX-2, IL-1β and IL-8. When co-cultured with mitogen-stimulated lymphocytes, ciMSCs downregulated their expression of iNOS, HGF, TGF-β1 and PTGER-2α, while increasing their expression of COX-2, IDO and IL-1β. Taken together, these findings suggest that ciMSCs possess similar immunomodulatory capabilities as harvested cMSCs and support further investigation into their potential use for the management of canine immune-mediated and inflammatory disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: a donor-matched comparative study.Stem Cell Res Ther. 2017 Oct 3;8(1):218. doi: 10.1186/s13287-017-0639-6. Stem Cell Res Ther. 2017. PMID: 28974260 Free PMC article.
-
Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells.Stem Cells Dev. 2008 Aug;17(4):681-93. doi: 10.1089/scd.2007.0153. Stem Cells Dev. 2008. PMID: 18717642
-
The lower in vitro chondrogenic potential of canine adipose tissue-derived mesenchymal stromal cells (MSC) compared to bone marrow-derived MSC is not improved by BMP-2 or BMP-6.Vet J. 2021 Mar;269:105605. doi: 10.1016/j.tvjl.2020.105605. Epub 2020 Dec 29. Vet J. 2021. PMID: 33593496
-
Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially.PLoS One. 2010 Feb 2;5(2):e9016. doi: 10.1371/journal.pone.0009016. PLoS One. 2010. PMID: 20126406 Free PMC article.
-
Therapeutic potential of adipose derived stromal cells for major skin inflammatory diseases.Front Med (Lausanne). 2024 Feb 23;11:1298229. doi: 10.3389/fmed.2024.1298229. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38463491 Free PMC article. Review.
Cited by
-
Effectiveness of RCTs Pooling Evidence on Mesenchymal Stem Cell (MSC) Therapeutic Applications During COVID-19 Epidemic: A Systematic Review.Biologics. 2023 May 18;17:85-112. doi: 10.2147/BTT.S404421. eCollection 2023. Biologics. 2023. PMID: 37223116 Free PMC article. Review.
-
The Intersection of Human and Veterinary Medicine-A Possible Direction towards the Improvement of Cell Therapy Protocols in the Treatment of Perianal Fistulas.Int J Mol Sci. 2022 Nov 11;23(22):13917. doi: 10.3390/ijms232213917. Int J Mol Sci. 2022. PMID: 36430390 Free PMC article. Review.
-
Comparative characteristic study from bone marrow-derived mesenchymal stem cells.J Vet Sci. 2021 Nov;22(6):e74. doi: 10.4142/jvs.2021.22.e74. Epub 2021 Aug 26. J Vet Sci. 2021. PMID: 34697921 Free PMC article. Review.
-
Mesenchymal stem cells and their derived exosomes to combat Covid-19.Rev Med Virol. 2022 Mar;32(2):e2281. doi: 10.1002/rmv.2281. Epub 2021 Aug 7. Rev Med Virol. 2022. PMID: 34363275 Free PMC article. Review.
-
The Pleiotropic role, functions and targeted therapies of LIF/LIFR axis in cancer: Old spectacles with new insights.Biochim Biophys Acta Rev Cancer. 2022 Jul;1877(4):188737. doi: 10.1016/j.bbcan.2022.188737. Epub 2022 Jun 6. Biochim Biophys Acta Rev Cancer. 2022. PMID: 35680099 Free PMC article. Review.
References
-
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

