A Phase I Clinical Study Comparing the Tolerance, Immunogenicity, and Pharmacokinetics of Proposed Biosimilar BAT1806 and Reference Tocilizumab in Healthy Chinese Men
- PMID: 33569002
- PMCID: PMC7868548
- DOI: 10.3389/fphar.2020.609522
A Phase I Clinical Study Comparing the Tolerance, Immunogenicity, and Pharmacokinetics of Proposed Biosimilar BAT1806 and Reference Tocilizumab in Healthy Chinese Men
Abstract
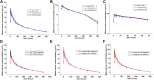
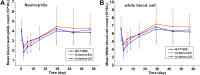
Objective: The study aimed to explore the bioequivalence of a proposed biosimilar BAT1806 to its reference products marketed in the EU and US (RoActemra-EU and Actemra-US) among healthy Chinese men. The tolerance, immunogenicity, and pharmacokinetics (PK) of the three drugs were also investigated. Methods: In this randomized, double-blind, single-dose, three-arm, parallel study, a single-dose of 4 mg/kg of the reference products, or the biosimilar was administered to the participants. The participants were followed up for 57 days, and PK, immunogenicity, and tolerance evaluations were completed during this period. Results: The PK parameters were similar in all three groups: BAT1806 (n = 45), RoActemra-EU (n = 42), and Actemra-US (n = 42). The 90% confidence intervals (CIs) for the ratios of C max , AUC0-t and AUC0-∞ were 86.90-104.41% for BAT1806 vs. RoActemra-EU, 91.70-106.15% for BAT1806 vs Actemra-US, and 90.04-105.53% for Actemra-US vs RoActemra-EU. For all comparisons, the 90% CIs for the C max , AUC0-t , and AUC0-∞ were within the predefined bioequivalence limit of 80.00-125.00%. The intersubject variability ranged from 14.5% to 21.5%, which was considerably low. Among the participants, 19 (42.2%), 10 (23.8%), and 12 (28.6%) from the BAT1806, RoActemra-EU, and Actemra-US groups were, respectively, found to be positive for anti-drug antibodies, while 14 (31.1%), nine (21.4%), and 12 (28.6%) were positive for neutralizing antibodies. Nevertheless, these antibodies did not affect the drug concentrations, and the outcomes in the bioequivalence tests were similar after sensitivity analysis. Treatment-related and treatment-emergent adverse events (TEAEs) were recorded in 27, 34, and 32 participants in the BAT1806, RoActemra-EU, and Actemra-US groups, respectively. The most common treatment-related adverse events observed were a decrease in neutrophil, and white blood cell counts. Conclusion: The PK characteristics of BAT1806 were similar to those of the reference products, RoActemra-EU and Actemra-US. Both BAT1806 and the reference products exhibited low intersubject variability and similar safety profiles. Clinical trial registration number: http://www.chinadrugtrials.org.cn/index.html, CTR20180039; https://clinicaltrials.gov/NCT03606876.
Keywords: and pharmacokinetics of tocilizumab biosimilar; biosimilar; immunogenicity; intersubject variability tolerance; pharmacokinetics; tocilizumab; variability.
Copyright © 2021 Zhang, Wang, Wei, Chen, Liu, Li, Zhu, Li, Yu, Zhou, Yang, Wang, Wu and Ding.
Conflict of interest statement
JY, YZ, XY and ZW were employed by the Bio-thera Solutions, Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abdallah H., Hsu J. C., Lu P., Fettner S., Zhang X., Douglass W., et al. (2017). Pharmacokinetic and pharmacodynamic analysis of subcutaneous tocilizumab in patients with rheumatoid arthritis from 2 randomized, controlled trials: SUMMACTA and BREVACTA. J. Clin. Pharmacol. 57, 459–468. 10.1002/jcph.826 - DOI - PMC - PubMed
-
- Administration C. F. a. D. (2015a). Scientific considerations in demonstrating bioequivalence to a reference product. Available at: http://www.sda.gov.cn/WS01/CL1751/147583.html (Accessed April 24, 2020).
-
- Administration U. S. F. a. D. (2015b). Scientific considerations in demonstrating biosimilarity to a reference product. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati... (Accessed May 1, 2015).
-
- Agency E. M. (2014). Guideline on similar biological medicinal products. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (Accessed August 25, 2015).
-
- Bastida C., Huitema A. D. R., l'Ami M. J., Ruiz-Esquide V., Wolbink G. J., Sanmartí R., et al. (2020). Evaluation of dose-tapering strategies for intravenous tocilizumab in rheumatoid arthritis patients using model-based pharmacokinetic/pharmacodynamic simulations. Eur. J. Clin. Pharmacol. 76, 1417–1425. 10.1007/s00228-020-02925-w - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

