Immune Privilege: The Microbiome and Uveitis
- PMID: 33569055
- PMCID: PMC7868421
- DOI: 10.3389/fimmu.2020.608377
Immune Privilege: The Microbiome and Uveitis
Abstract
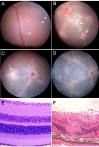
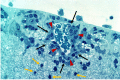
Immune privilege (IP), a term introduced to explain the unpredicted acceptance of allogeneic grafts by the eye and the brain, is considered a unique property of these tissues. However, immune responses are modified by the tissue in which they occur, most of which possess IP to some degree. The eye therefore displays a spectrum of IP because it comprises several tissues. IP as originally conceived can only apply to the retina as it contains few tissue-resident bone-marrow derived myeloid cells and is immunologically shielded by a sophisticated barrier - an inner vascular and an outer epithelial barrier at the retinal pigment epithelium. The vascular barrier comprises the vascular endothelium and the glia limitans. Immune cells do not cross the blood-retinal barrier (BRB) despite two-way transport of interstitial fluid, governed by tissue oncotic pressure. The BRB, and the blood-brain barrier (BBB) mature in the neonatal period under signals from the expanding microbiome and by 18 months are fully established. However, the adult eye is susceptible to intraocular inflammation (uveitis; frequency ~200/100,000 population). Uveitis involving the retinal parenchyma (posterior uveitis, PU) breaches IP, while IP is essentially irrelevant in inflammation involving the ocular chambers, uveal tract and ocular coats (anterior/intermediate uveitis/sclerouveitis, AU). Infections cause ~50% cases of AU and PU but infection may also underlie the pathogenesis of immune-mediated "non-infectious" uveitis. Dysbiosis accompanies the commonest form, HLA-B27-associated AU, while latent infections underlie BRB breakdown in PU. This review considers the pathogenesis of uveitis in the context of IP, infection, environment, and the microbiome.
Keywords: T regulatory cells; adjuvant effect; blood retinal barrier; commensals; folate; nutritional metabolites; probiotics.
Copyright © 2021 Mölzer, Heissigerova, Wilson, Kuffova and Forrester.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

