Calcium signaling: breast cancer's approach to manipulation of cellular circuitry
- PMID: 33569087
- PMCID: PMC7755621
- DOI: 10.1007/s12551-020-00771-9
Calcium signaling: breast cancer's approach to manipulation of cellular circuitry
Abstract
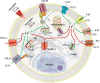
Calcium is a versatile element that participates in cell signaling for a wide range of cell processes such as death, cell cycle, division, migration, invasion, metabolism, differentiation, autophagy, transcription, and others. Specificity of calcium in each of these processes is achieved through modulation of intracellular calcium concentrations by changing the characteristics (amplitude/frequency modulation) or location (spatial modulation) of the signal. Breast cancer utilizes calcium signaling as an advantage for survival and progression. This review integrates evidence showing that increases in expression of calcium channels, GPCRs, pumps, effectors, and enzymes, as well as resulting intracellular calcium signals, lead to high calcium and/or an elevated calcium- mobilizing capacity necessary for malignant functions such as migratory, invasive, proliferative, tumorigenic, or metastatic capacities.
Keywords: Breast cancer; Calcium signaling.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no conflicts of interest.
Figures


References
-
- 2015 CRC handbook of chemistry and physics, 96th ed. CRC Press, 2015
-
- Abdoul-Azize S, Buquet C, Li H, Picquenot JM, Vannier JP. Integration of Ca(2+) signaling regulates the breast tumor cell response to simvastatin and doxorubicin. Oncogene. 2018;37:4979–4993. - PubMed
-
- Abdul M, Ramlal S, Hoosein N. Ryanodine receptor expression correlates with tumor grade in breast cancer. Pathol Oncol Res. 2008;14:157–160. - PubMed
-
- al-Mohanna FA, Caddy KW, Bolsover SR. The nucleus is insulated from large cytosolic calcium ion changes. Nature. 1994;367:745–750. - PubMed
-
- Anguita E, Villalobo A. Ca(2+) signaling and Src-kinases-controlled cellular functions. Arch Biochem Biophys. 2018;650:59–74. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

