Exploring a model-based analysis of patient derived xenograft studies in oncology drug development
- PMID: 33569251
- PMCID: PMC7847196
- DOI: 10.7717/peerj.10681
Exploring a model-based analysis of patient derived xenograft studies in oncology drug development
Abstract
Purpose: To assess whether a model-based analysis increased statistical power over an analysis of final day volumes and provide insights into more efficient patient derived xenograft (PDX) study designs.
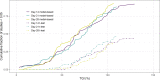
Methods: Tumour xenograft time-series data was extracted from a public PDX drug treatment database. For all 2-arm studies the percent tumour growth inhibition (TGI) at day 14, 21 and 28 was calculated. Treatment effect was analysed using an un-paired, two-tailed t-test (empirical) and a model-based analysis, likelihood ratio-test (LRT). In addition, a simulation study was performed to assess the difference in power between the two data-analysis approaches for PDX or standard cell-line derived xenografts (CDX).
Results: The model-based analysis had greater statistical power than the empirical approach within the PDX data-set. The model-based approach was able to detect TGI values as low as 25% whereas the empirical approach required at least 50% TGI. The simulation study confirmed the findings and highlighted that CDX studies require fewer animals than PDX studies which show the equivalent level of TGI.
Conclusions: The study conducted adds to the growing literature which has shown that a model-based analysis of xenograft data improves statistical power over the common empirical approach. The analysis conducted showed that a model-based approach, based on the first mathematical model of tumour growth, was able to detect smaller size of effect compared to the empirical approach which is common of such studies. A model-based analysis should allow studies to reduce animal use and experiment length providing effective insights into compound anti-tumour activity.
Keywords: Mathematical modelling; Patient derived xenograft; Statistical modelling; Tumour growth inhibition.
©2021 Dickinson et al.
Conflict of interest statement
Marcel de Matas and Paul A. Dickinson are Directors and Shareholders; Jake Dickinson is an employee and Hitesh B. Mistry is a contractor of Seda Pharmaceutical Development Services Ltd. which provides modelling services to the Pharmaceutical Industry.
Figures
References
-
- Corbett T, Polin L, LoRusso P, Valeriote F, Panchapor C, Pugh S, White K, Knight J, Demchik L, Jones J, Jones L, Lisow L. In Vivo methods for screening and preclinical testing. In: Teicher BA, Andrews PA, editors. Anticancer drug development guide: preclinical screening, clinical trials, and approval. Humana Press; Totowa: 2004. pp. 99–123.
-
- Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, Zhang C, Schnell C, Yang G, Zhang Y, Balbin OA, Barbe S, Cai H, Casey F, Chatterjee S, Chiang DY, Chuai S, Cogan SM, Collins SD, Dammassa E, Ebel N, Embry M, Green J, Kauffmann A, Kowal C, Leary RJ, Lehar J, Liang Y, Loo A, Lorenzana E, McDonald III RE, McLaughlin ME, Merkin J, Meyer R, Naylor TL, Patawaran M, Reddy A, Röelli C, Ruddy DA, Salangsang F, Santacroce F, Singh AP, Tang Y, Tinetto W, Tobler S, Velazquez R, Venkatesan K, Von Arx F, Wang HQ, Wang Z, Wiesmann M, Wyss D, Xu F, Bitter H, Atadja P, Lees E, Hofmann F, Li E, Keen N, Cozens R, Jensen MR, Pryer NK, Williams JA, William R, Sellers WR. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nature Medicine. 2015;21(11):1318–1325. doi: 10.1038/nm.3954. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources