The Roles of Epigenetics Regulation in Bone Metabolism and Osteoporosis
- PMID: 33569383
- PMCID: PMC7868402
- DOI: 10.3389/fcell.2020.619301
The Roles of Epigenetics Regulation in Bone Metabolism and Osteoporosis
Abstract
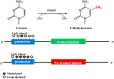
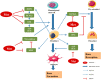
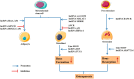
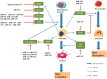
Osteoporosis is a metabolic disease characterized by decreased bone mineral density and the destruction of bone microstructure, which can lead to increased bone fragility and risk of fracture. In recent years, with the deepening of the research on the pathological mechanism of osteoporosis, the research on epigenetics has made significant progress. Epigenetics refers to changes in gene expression levels that are not caused by changes in gene sequences, mainly including DNA methylation, histone modification, and non-coding RNAs (lncRNA, microRNA, and circRNA). Epigenetics play mainly a post-transcriptional regulatory role and have important functions in the biological signal regulatory network. Studies have shown that epigenetic mechanisms are closely related to osteogenic differentiation, osteogenesis, bone remodeling and other bone metabolism-related processes. Abnormal epigenetic regulation can lead to a series of bone metabolism-related diseases, such as osteoporosis. Considering the important role of epigenetic mechanisms in the regulation of bone metabolism, we mainly review the research progress on epigenetic mechanisms (DNA methylation, histone modification, and non-coding RNAs) in the osteogenic differentiation and the pathogenesis of osteoporosis to provide a new direction for the treatment of bone metabolism-related diseases.
Keywords: DNA methylation; epigenetics; histone modification; non-coding RNA; osteoporosis.
Copyright © 2021 Xu, Li, Yang, Na, Chen and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

