Pyrotinib versus trastuzumab emtansine for HER2-positive metastatic breast cancer after previous trastuzumab and lapatinib treatment: a real-world study
- PMID: 33569405
- PMCID: PMC7867920
- DOI: 10.21037/atm-20-4054
Pyrotinib versus trastuzumab emtansine for HER2-positive metastatic breast cancer after previous trastuzumab and lapatinib treatment: a real-world study
Abstract
Background: To compare the efficacy and safety of pyrotinib and trastuzumab emtansine (T-DM1) in patients who experienced disease progression on trastuzumab and lapatinib treatment.
Methods: This was a real-world study that included cases of metastatic breast cancer (MBC) with trastuzumab and lapatinib failure. One group of patients received pyrotinib monotherapy or combination therapy, whereas the other group received T-DM1 monotherapy. The primary study endpoint was progression-free survival (PFS); secondary endpoints were the objective response rate (ORR), clinical benefit rate (CBR) and safety.
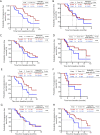
Results: Between January 2013 and November 2019, 105 patients were enrolled in the pyrotinib group (n=55) or T-DM1 group (n=50). The median PFS was 6.0 months (95% CI, 4.7 to 7.3 months) with pyrotinib and 4.2 months (95% CI, 3.6 to 4.8 months) with T-DM1 (P=0.044). ORR values were 16.3% and 20.0% in the pyrotinib and T-DM1 groups, respectively (P=0.629); CBR values were 45.5% and 40.0% in the pyrotinib and T-DM1 groups, respectively (P=0.573). Subgroup analysis of those benefitting from lapatinib revealed a median PFS of 8.1 months (95% CI, 4.8 to 11.4 months) in the pyrotinib group, whereas that of the T-DM1 group was 4.4 months (95% CI, 3.8 to 5.0 months, P=0.013). Moreover, the median PFS of patients without liver metastases was 6.9 months (95% CI, 3.7 to 10.1 months) in the pyrotinib group and 4.1 months (95% CI, 3.1 to 5.1 months) in the T-DM1 group (P=0.010). The main common adverse events (AEs) were diarrhea (98.2%) and nausea (49.1%) in the pyrotinib group and thrombocytopenia (42.0%) and nausea (40.0%) in the T-DM1 group. The percentages of grade 3 to 4 AEs in the pyrotinib and T-DM1 groups were 34.5% and 40.0%, respectively.
Conclusions: The results of this study suggest that patients with HER2-positive MBC with trastuzumab and lapatinib failure can benefit from subsequent pyrotinib treatment and tolerate this treatment well, especially those who have benefited from previous lapatinib treatment or those who have no liver metastasis.
Keywords: HER2; Metastatic breast cancer (MBC); T-DM1; pyrotinib.
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4054). The authors have no conflicts of interest to declare.
Figures

References
-
- Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011;29:3366-73. 10.1200/JCO.2011.35.0868 - DOI - PMC - PubMed
-
- NCCN . Clinical practice guidelines in oncology (NCCN guidelines), breast Cancer 2020.
-
- Li J, Jiang Z. CSCO BC guideline: updates for HER2 positive breast cancer in 2020. Transl Breast Cancer Res 2020;1:4. 10.21037/tbcr.2020.03.05 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
