Two decades since the fetal insulin hypothesis: what have we learned from genetics?
- PMID: 33569631
- PMCID: PMC7940336
- DOI: 10.1007/s00125-021-05386-7
Two decades since the fetal insulin hypothesis: what have we learned from genetics?
Abstract
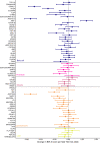
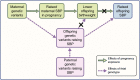
In 1998 the fetal insulin hypothesis proposed that lower birthweight and adult-onset type 2 diabetes are two phenotypes of the same genotype. Since then, advances in research investigating the role of genetics affecting insulin secretion and action have furthered knowledge of fetal insulin-mediated growth and the biology of type 2 diabetes. In this review, we discuss the historical research context from which the fetal insulin hypothesis originated and consider the position of the hypothesis in light of recent evidence. In summary, there is now ample evidence to support the idea that variants of certain genes which result in impaired pancreatic beta cell function and reduced insulin secretion contribute to both lower birthweight and higher type 2 diabetes risk in later life when inherited by the fetus. There is also evidence to support genetic links between type 2 diabetes secondary to reduced insulin action and lower birthweight but this applies only to loci implicated in body fat distribution and not those influencing insulin resistance via obesity or lipid metabolism by the liver. Finally, we also consider how advances in genetics are being used to explore alternative hypotheses, namely the role of the maternal intrauterine environment, in the relationship between lower birthweight and adult cardiometabolic disease.
Keywords: Birthweight; Fetal growth restriction; Fetal insulin; Genome-wide association studies; Mendelian randomisation; Neonatal diabetes; Pregnancy; Review; Type 2 diabetes.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

