Challenges for Targeting SARS-CoV-2 Proteases as a Therapeutic Strategy for COVID-19
- PMID: 33570381
- PMCID: PMC7901237
- DOI: 10.1021/acsinfecdis.0c00815
Challenges for Targeting SARS-CoV-2 Proteases as a Therapeutic Strategy for COVID-19
Abstract
Two proteases produced by the SARS-CoV-2 virus, the main protease and papain-like protease, are essential for viral replication and have become the focus of drug development programs for treatment of COVID-19. We screened a highly focused library of compounds containing covalent warheads designed to target cysteine proteases to identify new lead scaffolds for both Mpro and PLpro proteases. These efforts identified a small number of hits for the Mpro protease and no viable hits for the PLpro protease. Of the Mpro hits identified as inhibitors of the purified recombinant protease, only two compounds inhibited viral infectivity in cellular infection assays. However, we observed a substantial drop in antiviral potency upon expression of TMPRSS2, a transmembrane serine protease that acts in an alternative viral entry pathway to the lysosomal cathepsins. This loss of potency is explained by the fact that our lead Mpro inhibitors are also potent inhibitors of host cell cysteine cathepsins. To determine if this is a general property of Mpro inhibitors, we evaluated several recently reported compounds and found that they are also effective inhibitors of purified human cathepsins L and B and showed similar loss in activity in cells expressing TMPRSS2. Our results highlight the challenges of targeting Mpro and PLpro proteases and demonstrate the need to carefully assess selectivity of SARS-CoV-2 protease inhibitors to prevent clinical advancement of compounds that function through inhibition of a redundant viral entry pathway.
Keywords: SARS-CoV-2; cathepsin cross-reactivity; main protease; papain-like protease; viral entry.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests
Figures
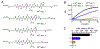



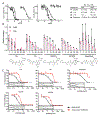
References
-
- Zhou P; Yang X; Lou; Wang XG; Hu B; Zhang L; Zhang W; Si HR; Zhu Y; Li B; Huang CL; Chen HD; Chen J; Luo Y; Guo H; Jiang R. Di; Liu MQ; Chen Y; Shen XR; Wang X; Zheng XS; Zhao K; Chen QJ; Deng F; Liu LL; Yan B; Zhan FX; Wang YY; Xiao GF; Shi ZL A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579 (7798), 270–273. 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Jin Z; Du X; Xu Y; Deng Y; Liu M; Zhao Y; Zhang B; Li X; Zhang L; Peng C; Duan Y; Yu J; Wang L; Yang K; Liu F; Jiang R; Yang X; You T; Liu X; Yang X; Bai F; Liu H; Liu X; Guddat LW; Xu W; Xiao G; Qin C; Shi Z; Jiang H; Rao Z; Yang H Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors. Nature 2020, 582 (7811), 289–293. 10.1038/s41586-020-2223-y. - DOI - PubMed
-
- Yang H; Xie W; Xue X; Yang K; Ma J; Liang W; Zhao Q; Zhou Z; Pei D; Ziebuhr J; Hilgenfeld R; Kwok YY; Wong L; Gao G; Chen S; Chen Z; Ma D; Bartlam M; Rao Z Design of Wide-Spectrum Inhibitors Targeting Coronavirus Main Proteases. PLoS Biol 2005, 3 (10). 10.1371/journal.pbio.0030324. - DOI - PMC - PubMed
-
- Dai W; Zhang B; Jiang XM; Su H; Li J; Zhao Y; Xie X; Jin Z; Peng J; Liu F; Li C; Li Y; Bai F; Wang H; Cheng X; Cen X; Hu S; Yang X; Wang J; Liu X; Xiao G; Jiang H; Rao Z; Zhang LK; Xu Y; Yang H; Liu H Structure-Based Design of Antiviral Drug Candidates Targeting the SARS-CoV-2 Main Protease. Science (80- ) 2020, 368 (6497), 1331–1335. 10.1126/science.abb4489. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

