Carotid body chemoreceptors: physiology, pathology, and implications for health and disease
- PMID: 33570461
- PMCID: PMC8526340
- DOI: 10.1152/physrev.00039.2019
Carotid body chemoreceptors: physiology, pathology, and implications for health and disease
Abstract
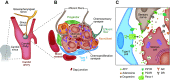
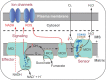
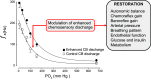
The carotid body (CB) is the main peripheral chemoreceptor for arterial respiratory gases O2 and CO2 and pH, eliciting reflex ventilatory, cardiovascular, and humoral responses to maintain homeostasis. This review examines the fundamental biology underlying CB chemoreceptor function, its contribution to integrated physiological responses, and its role in maintaining health and potentiating disease. Emphasis is placed on 1) transduction mechanisms in chemoreceptor (type I) cells, highlighting the role played by the hypoxic inhibition of O2-dependent K+ channels and mitochondrial oxidative metabolism, and their modification by intracellular molecules and other ion channels; 2) synaptic mechanisms linking type I cells and petrosal nerve terminals, focusing on the role played by the main proposed transmitters and modulatory gases, and the participation of glial cells in regulation of the chemosensory process; 3) integrated reflex responses to CB activation, emphasizing that the responses differ dramatically depending on the nature of the physiological, pathological, or environmental challenges, and the interactions of the chemoreceptor reflex with other reflexes in optimizing oxygen delivery to the tissues; and 4) the contribution of enhanced CB chemosensory discharge to autonomic and cardiorespiratory pathophysiology in obstructive sleep apnea, congestive heart failure, resistant hypertension, and metabolic diseases and how modulation of enhanced CB reactivity in disease conditions may attenuate pathophysiology.
Keywords: autonomic system; carotid body; hypoxia.
Conflict of interest statement
V.K.S. has served as a consultant for Respicardia, Roche, and Bayer. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures











References
-
- Fitzgerald RS. The carotid body: terrestrial mammals' most important peripheral neuroreceptor? J Neurol Neurophysiol 7: 1000407, 2016. doi: 10.4172/2155-9562.1000407. - DOI
-
- Hering HE. Ueber die wand des sinus caroticus als reizempfänger und den sinusnerv als zentripetale bahn für die sinusreflexe. Dtsch med Wochenschr 51: 1140–1141, 1925. doi: 10.1055/s-0028-1136917. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

