The Spike D614G mutation increases SARS-CoV-2 infection of multiple human cell types
- PMID: 33570490
- PMCID: PMC7891930
- DOI: 10.7554/eLife.65365
The Spike D614G mutation increases SARS-CoV-2 infection of multiple human cell types
Abstract
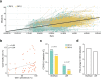
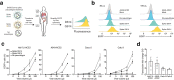
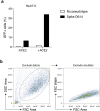
A novel variant of the SARS-CoV-2 virus carrying a point mutation in the Spike protein (D614G) has recently emerged and rapidly surpassed others in prevalence. This mutation is in linkage disequilibrium with an ORF1b protein variant (P314L), making it difficult to discern the functional significance of the Spike D614G mutation from population genetics alone. Here, we perform site-directed mutagenesis on wild-type human-codon-optimized Spike to introduce the D614G variant. Using multiple human cell lines, including human lung epithelial cells, we found that the lentiviral particles pseudotyped with Spike D614G are more effective at transducing cells than ones pseudotyped with wild-type Spike. The increased transduction with Spike D614G ranged from 1.3- to 2.4-fold in Caco-2 and Calu-3 cells expressing endogenous ACE2 and from 1.5- to 7.7-fold in A549ACE2 and Huh7.5ACE2 overexpressing ACE2. Furthermore, trans-complementation of SARS-CoV-2 virus with Spike D614G showed an increased infectivity in human cells. Although there is minimal difference in ACE2 receptor binding between the D614 and G614 Spike variants, the G614 variant is more resistant to proteolytic cleavage, suggesting a possible mechanism for the increased transduction.
Keywords: COVID-19; D614G; SARS-CoV-2; Spike; coronavirus; epidemiology; global health; human; infectious disease; microbiology; virus.
© 2021, Daniloski et al.
Conflict of interest statement
ZD, TJ, JI, XG, GB, Bt No competing interests declared, NS N.E.S. is an advisor to Vertex.
Figures






Update of
-
The Spike D614G mutation increases SARS-CoV-2 infection of multiple human cell types.bioRxiv [Preprint]. 2020 Jul 7:2020.06.14.151357. doi: 10.1101/2020.06.14.151357. bioRxiv. 2020. Update in: Elife. 2021 Feb 11;10:e65365. doi: 10.7554/eLife.65365. PMID: 32587969 Free PMC article. Updated. Preprint.
References
-
- Bestle D, Heindl MR, Limburg H, Van TVL, Pilgram O, Moulton H, Stein DA, Hardes K, Eickmann M, Dolnik O, Rohde C, Becker S, Klenk H-D, Garten W, Steinmetzer T, Böttcher-Friebertshäuser E. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. bioRxiv. 2020 doi: 10.1101/2020.04.15.042085. - DOI - PMC - PubMed
-
- Bhattacharyya C, Das C, Ghosh A, Singh AK, Mukherjee S, Majumder PP, Basu A, Biswas NK. Global spread of SARS-CoV-2 subtype with spike protein mutation D614G is shaped by human genomic variations that regulate expression of TMPRSS2 and MX1 genes. bioRxiv. 2020 doi: 10.1101/2020.05.04.075911. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI123155/AI/NIAID NIH HHS/United States
- R01 CA218668/CA/NCI NIH HHS/United States
- 20POST35220040/American Heart Association
- DP2 HG010099/HG/NHGRI NIH HHS/United States
- R00 HG008171/HG/NHGRI NIH HHS/United States
- R01AI147131/National Institute of Allergy and Infectious Diseases
- R01 AI147131/AI/NIAID NIH HHS/United States
- R00HG008171/GF/NIH HHS/United States
- HR0011-20-2-0040/Defense Advanced Research Projects Agency
- R01AI123155/National Institute of Allergy and Infectious Diseases
- DP2HG010099/HG/NHGRI NIH HHS/United States
- R01CA218668/CA/NCI NIH HHS/United States
- PEW-00033055/Pew Charitable Trusts
- D18AP00053/Defense Advanced Research Projects Agency
- SSP-2018-2737/Searle Scholars Program
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

