An Interactive Voice Response Software to Improve the Quality of Life of People Living With HIV in Uganda: Randomized Controlled Trial
- PMID: 33570497
- PMCID: PMC7906832
- DOI: 10.2196/22229
An Interactive Voice Response Software to Improve the Quality of Life of People Living With HIV in Uganda: Randomized Controlled Trial
Abstract
Background: Following the successful scale-up of antiretroviral therapy (ART), the focus is now on ensuring good quality of life (QoL) and sustained viral suppression in people living with HIV. The access to mobile technology in the most burdened countries is increasing rapidly, and therefore, mobile health (mHealth) technologies could be leveraged to improve QoL in people living with HIV. However, data on the impact of mHealth tools on the QoL in people living with HIV are limited to the evaluation of SMS text messaging; these are infeasible in high-illiteracy settings.
Objective: The primary and secondary outcomes were to determine the impact of interactive voice response (IVR) technology on Medical Outcomes Study HIV QoL scores and viral suppression at 12 months, respectively.
Methods: Within the Call for Life study, ART-experienced and ART-naïve people living with HIV commencing ART were randomized (1:1 ratio) to the control (no IVR support) or intervention arm (daily adherence and pre-appointment reminders, health information tips, and option to report symptoms). The software evaluated was Call for Life Uganda, an IVR technology that is based on the Mobile Technology for Community Health open-source software. Eligibility criteria for participation included access to a phone, fluency in local languages, and provision of consent. The differences in differences (DIDs) were computed, adjusting for baseline HIV RNA and CD4.
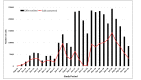
Results: Overall, 600 participants (413 female, 68.8%) were enrolled and followed-up for 12 months. In the intervention arm of 300 participants, 298 (99.3%) opted for IVR and 2 (0.7%) chose SMS text messaging as the mode of receiving reminders and health tips. At 12 months, there was no overall difference in the QoL between the intervention and control arms (DID=0.0; P=.99) or HIV RNA (DID=0.01; P=.94). At 12 months, 124 of the 256 (48.4%) active participants had picked up at least 50% of the calls. In the active intervention participants, high users (received >75% of reminders) had overall higher QoL compared to low users (received <25% of reminders) (92.2 versus 87.8, P=.02). Similarly, high users also had higher QoL scores in the mental health domain (93.1 versus 86.8, P=.008) and better appointment keeping. Similarly, participants with moderate use (51%-75%) had better viral suppression at 12 months (80/94, 85% versus 11/19, 58%, P=.006).
Conclusions: Overall, there was high uptake and acceptability of the IVR tool. While we found no overall difference in the QoL and viral suppression between study arms, people living with HIV with higher usage of the tool showed greater improvements in QoL, viral suppression, and appointment keeping. With the declining resources available to HIV programs and the increasing number of people living with HIV accessing ART, IVR technology could be used to support patient care. The tool may be helpful in situations where physical consultations are infeasible, including the current COVID epidemic.
Trial registration: ClinicalTrials.gov NCT02953080; https://clinicaltrials.gov/ct2/show/NCT02953080.
Keywords: HIV; digital health; interactive voice response; mHealth; mobile health; quality of life.
©Dathan Mirembe Byonanebye, Maria S Nabaggala, Agnes Bwanika Naggirinya, Mohammed Lamorde, Elizabeth Oseku, Rachel King, Noela Owarwo, Eva Laker, Richard Orama, Barbara Castelnuovo, Agnes Kiragga, Rosalind Parkes-Ratanshi. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 11.02.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- 2019 Global HIV Statistics. Geneva: UN Joint Programme on HIV/AIDS (UNAIDS); 2020. [2020-11-08]. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_....
-
- Lifson AR, Grund B, Gardner EM, Kaplan R, Denning E, Engen N, Carey CL, Chen F, Dao S, Florence E, Sanz J, Emery S, INSIGHT START Study Group Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection. AIDS. 2017 Apr 24;31(7):953–963. doi: 10.1097/QAD.0000000000001417. http://europepmc.org/abstract/MED/28121710 - DOI - PMC - PubMed
-
- Mannheimer SB, Matts J, Telzak E, Chesney M, Child C, Wu AW, Friedland G, Terry Beirn Community Programs for Clinical Research on AIDS Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2005 Jan;17(1):10–22. doi: 10.1080/09540120412331305098. - DOI - PubMed
-
- Miners A, Phillips A, Kreif N, Rodger A, Speakman A, Fisher M, Anderson J, Collins S, Hart G, Sherr L, Lampe FC, ASTRA (Antiretrovirals‚ Sexual Transmission and Attitudes) Study Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. Lancet HIV. 2014 Oct;1(1):e32–40. doi: 10.1016/S2352-3018(14)70018-9. https://linkinghub.elsevier.com/retrieve/pii/S2352-3018(14)70018-9 - DOI - PubMed
-
- World Health Organization . Global Health Sector Strategy on HIV, 2016–2021: Towards Ending AIDS. Geneva: World Health Organization; [2020-11-02]. Building an Investment Case. https://apps.who.int/iris/bitstream/handle/10665/246178/WHO-HIV-2016.05-....
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

