Polarized mitochondria as guardians of NK cell fitness
- PMID: 33570622
- PMCID: PMC7805327
- DOI: 10.1182/bloodadvances.2020003458
Polarized mitochondria as guardians of NK cell fitness
Abstract
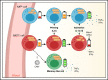
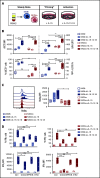
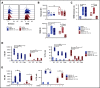
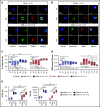
Distinct metabolic demands accompany lymphocyte differentiation into short-lived effector and long-lived memory cells. How bioenergetics processes are structured in innate natural killer (NK) cells remains unclear. We demonstrate that circulating human CD56Dim (NKDim) cells have fused mitochondria and enhanced metabolism compared with CD56Br (NKBr) cells. Upon activation, these 2 subsets showed a dichotomous response, with further mitochondrial potentiation in NKBr cells vs paradoxical mitochondrial fission and depolarization in NKDim cells. The latter effect impaired interferon-γ production, but rescue was possible by inhibiting mitochondrial fragmentation, implicating mitochondrial polarization as a central regulator of NK cell function. NKDim cells are heterogeneous, and mitochondrial polarization was associated with enhanced survival and function in mature NKDim cells, including memory-like human cytomegalovirus-dependent CD57+NKG2C+ subsets. In contrast, patients with genetic defects in mitochondrial fusion had a deficiency in adaptive NK cells, which had poor survival in culture. These results support mitochondrial polarization as a central regulator of mature NK cell fitness.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Bantug GR, Galluzzi L, Kroemer G, Hess C. The spectrum of T cell metabolism in health and disease. Nat Rev Immunol. 2018;18(1):19-34. - PubMed

