Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma
- PMID: 33570626
- PMCID: PMC7805341
- DOI: 10.1182/bloodadvances.2020002732
Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma
Abstract
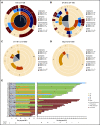
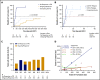
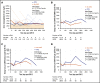
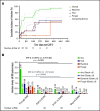
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 has significantly improved outcomes in the treatment of refractory or relapsed large B-cell lymphoma (LBCL). We evaluated the long-term course of hematologic recovery, immune reconstitution, and infectious complications in 41 patients with LBCL treated with axicabtagene ciloleucel (axi-cel) at a single center. Grade 3+ cytopenias occurred in 97.6% of patients within the first 28 days postinfusion, with most resolved by 6 months. Overall, 63.4% of patients received a red blood cell transfusion, 34.1% of patients received a platelet transfusion, 36.6% of patients received IV immunoglobulin, and 51.2% of patients received growth factor (granulocyte colony-stimulating factor) injections beyond the first 28 days postinfusion. Only 40% of patients had recovered detectable CD19+ B cells by 1 year, and 50% of patients had a CD4+ T-cell count <200 cells per μL by 18 months postinfusion. Patients with durable responses to axi-cel had significantly longer durations of B-cell aplasia, and this duration correlated strongly with the recovery of CD4+ T-cell counts. There were significantly more infections within the first 28 days compared with any other period of follow-up, with the majority being mild-moderate in severity. Receipt of corticosteroids was the only factor that predicted risk of infection in a multivariate analysis (hazard ratio, 3.69; 95% confidence interval, 1.18-16.5). Opportunistic infections due to Pneumocystis jirovecii and varicella-zoster virus occurred up to 18 months postinfusion in patients who prematurely discontinued prophylaxis. These results support the use of comprehensive supportive care, including long-term monitoring and antimicrobial prophylaxis, beyond 12 months after axi-cel treatment.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.S. has received research support from Kite Pharma-Gilead. A.R.R. has received research support from Pharmacyclics/AbbVie; has served on ad hoc scientific advisory boards for Nohla Therapeutics and Kaleido; has served as an expert witness for US Department of Justice; and his brother works for Johnson & Johnson. T.L. has been a member of the speaker’s bureau for Kite Pharma-Gilead. C.L.M. has acted as a consultant for Lyell, NeoimmuneTech, Nektar Therapeutics, and Apricity Therapeutics; has received royalties from the National Institutes of Heath and Juno Therapeutics for CD22-CARl and has equity in Lyell and Allogene Therapeutics. D.B.M. has acted as a consulting for Kite Pharma-Gilead, Juno Therapeutics-Celgene, Novartis, Janssen Pharmaceuticals, and Pharmacyclics and has received research support from Kite Pharma-Gilead, Allogene Therapeutics, Pharmacyclics, Miltenyi Biotec, and Adaptive Biotechnologies. S.S. has acted as a consultant for Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures


References
-
- Neelapu SS, Rossi JM, Jacobson CA, et al. CD19-loss with preservation of other B cell lineage features in patients with large B cell lymphoma who relapsed post-axi-cel. Blood. 2019;134(suppl 1):203.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

