αβ T-cell graft depletion for allogeneic HSCT in adults with hematological malignancies
- PMID: 33570642
- PMCID: PMC7805311
- DOI: 10.1182/bloodadvances.2020002444
αβ T-cell graft depletion for allogeneic HSCT in adults with hematological malignancies
Abstract
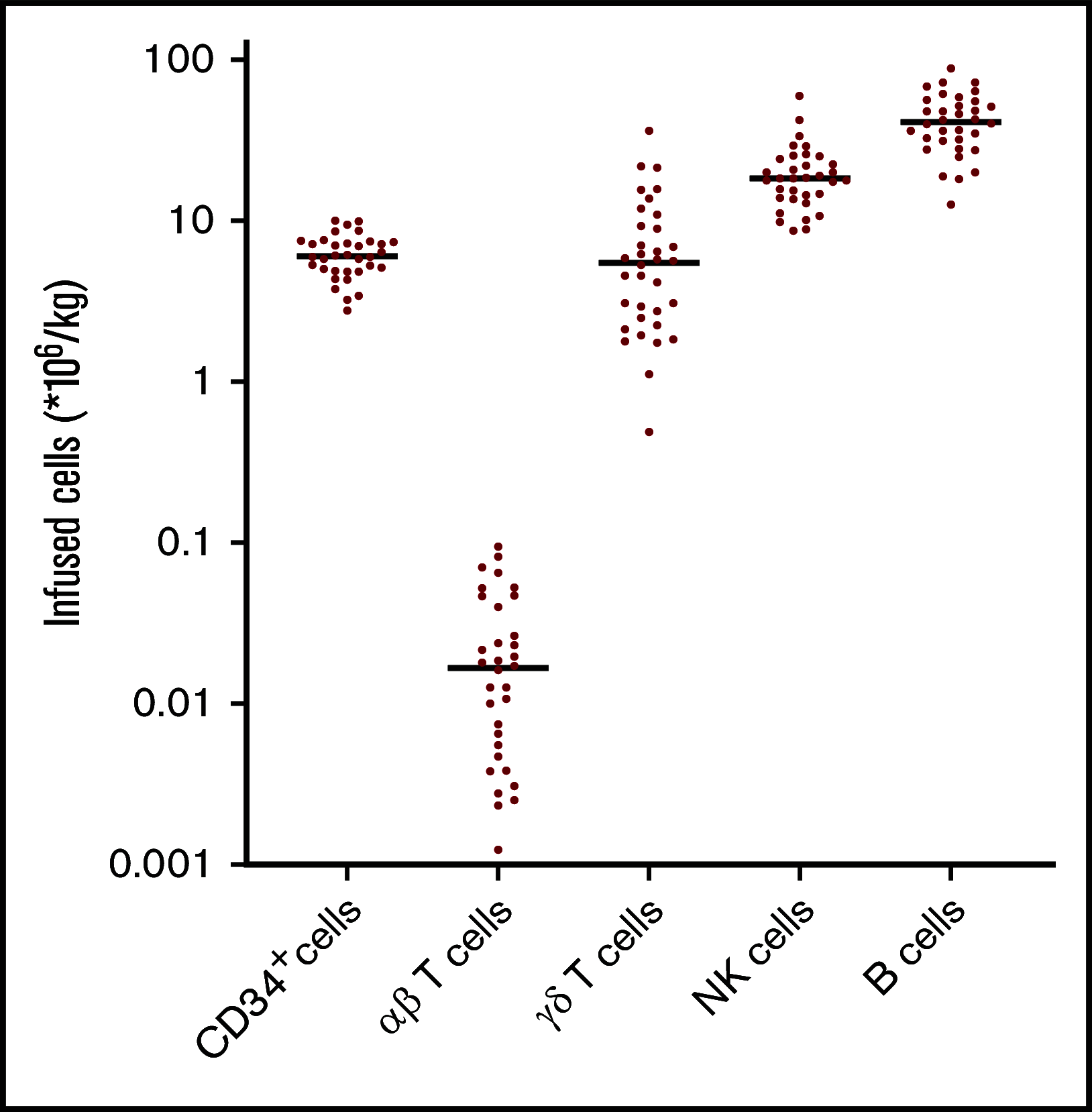
We conducted a multicenter prospective single-arm phase 1/2 study that assesses the outcome of αβ T-cell depleted allogeneic hematopoietic stem cell transplantation (allo-HSCT) of peripheral blood derived stem cells from matched related, or unrelated donors (10/10 and 9/10) in adults, with the incidence of acute graft-versus-host disease (aGVHD) as the primary end point at day 100. Thirty-five adults (median age, 59; range, 19-69 years) were enrolled. Conditioning consisted of antithymocyte globulin, busulfan, and fludarabine, followed by 28 days of mycophenolic acid after allo-HSCT. The minimal follow-up time was 24 months. The median number of infused CD34+ cells and αβ T cells were 6.1 × 106 and 16.3 × 103 cells per kg, respectively. The cumulative incidence (CI) of aGVHD grades 2-4 and 3-4 at day 100 was 26% and 14%. One secondary graft failure was observed. A prophylactic donor lymphocyte infusion (DLI) (1 × 105 CD3+ T cells per kg) was administered to 54% of the subjects, resulting in a CI of aGVHD grades 2-4 and 3-4 to 37% and 17% at 2 years. Immune monitoring revealed an early reconstitution of natural killer (NK) and γδ T cells. Cytomegalovirus reactivation associated with expansion of memory-like NK cells. The CI of relapse was 29%, and the nonrelapse mortality 32% at 2 years. The 2-year CI of chronic GVHD (cGVHD) was 23%, of which 17% was moderate. We conclude that only 26% of patients developed aGVHD 2-4 after αβ T-cell-depleted allo-HSCT within 100 days and was associated with a low incidence of cGVHD after 2 years. This trial was registered at www.trialregister.nl as #NL4767.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.-J.B. reports honoraria received from Avrobio, Magenta, Advanced Clinical, Takeda, and Bluerock for consulting. J.K. reports grants from Gadeta, Novartis, and Miltenyi Biotech and is inventor on patents dealing with γδ T-cell–related aspects as well as cofounder and shareholder of Gadeta. The remaining authors declare no competing financial interests.
Figures



References
-
- Frederik Falkenburg JH, Schmid C, Kolb HJ, Locatelli F, Kuball J. Delayed transfer of immune cells or the art of donor lymphocyte infusion In: Carreras E, Dufour C, Mohty M, Kroger N, eds. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, Cham, Switzerland: Springer; 2019:443-448. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

