Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection
- PMID: 33570644
- PMCID: PMC7802524
- DOI: 10.1182/bloodadvances.2020003456
Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection
Abstract
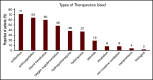
We aimed to identify predictors of outcomes and survival in patients living in 4 major metropolitan areas who had sickle cell disease (SCD) and COVID-19 to inform best approaches to prevention and care. Data were collected at baseline and during the clinical course in SCD patients diagnosed with COVID-19 in four COVID-19 epicenters. Patients were followed up posthospital discharge for up to 3 months. Of sixty-six SCD patients with COVID-19, fifty patients (75%) required hospitalization, and seven died (10.6%). Patients with preexisting kidney disease (chronic kidney disease) were more likely to be hospitalized. The most common presenting symptom was vaso-occlusive pain. Acute chest syndrome occurred in 30 (60%) of the 50 hospitalized patients and in all who died. Older age and histories of pulmonary hypertension, congestive heart failure, chronic kidney disease, and stroke were more prevalent in patients who died, as were higher creatinine, lactate dehydrogenase, and D-dimer levels. Anticoagulation use while inpatient was twice less common in patients who died. All deaths occurred in individuals not taking hydroxyurea or any other SCD-modifying therapy. Patients with SCD and COVID-19 exhibited a broad range of disease severity. We cannot definitively state that the overall mortality is higher in patients with SCD, although our case fatality rate was ∼10% compared with ∼3% in the general population, despite a median age of 34 years. Individuals with SCD aged >50 years, with preexisting cardiopulmonary, renal disease, and/or stroke not receiving hydroxyurea, who present with high serum creatinine, lactate dehydrogenase, and D-dimer levels, are at higher risk of death, irrespective of genotype or sex.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.P.M. declares honoraria for consulting/advisory boards for GBT, Emmaus, Roche, Forma, Novartis, CSL Behring, Bluebird Bio, and research funds from GBT. A.U.Z. declares honoraria/advisory boards from Global Blood Therapeutics, Novartis, Emmaus Life Sciences, Cyclerion, Imara, and Speakers Bureau: Global Blood Therapeutics. M.U.C. declares grants and personal fees from Bayer, Biomarin, Bluebird Bio, Global Blood Therapeutics, Hema Biologics, Kedrion, Octapharma, Pfizer, Roche/Genentech, Sanofi/Bioverativ, Spark Therapeutics, and Takeda. J.G. delcares Eli Lilly research funding, and Global Blood Therapeutics consulting. E.S.K. declares research support from Bayer and Arena/United Therapeutics, consulting for Novartis, Micelle Data and Safety Monitoring Board for the Phase III trial of Omega 3 Fatty Acids for Children and Adolescents with Sickle Cell Disease, and Novartis development of a disease severity score for patients with sickle cell disease. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Thrombocytopenia in a teen with sickle cell disease following COVID-19 vaccination.Pediatr Blood Cancer. 2021 Dec;68(12):e29271. doi: 10.1002/pbc.29271. Epub 2021 Jul 31. Pediatr Blood Cancer. 2021. PMID: 34331506 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention Provisional Death Counts for Coronavirus Disease. Daily Updates of Totals by Week and State. 2019. (COVID-19). https://www.cdc.gov/nchs/nvss/vsrr/COVID19/index.htm. Accessed 1 June 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

