The use of luspatercept for thalassemia in adults
- PMID: 33570654
- PMCID: PMC7805339
- DOI: 10.1182/bloodadvances.2020002725
The use of luspatercept for thalassemia in adults
Abstract
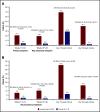
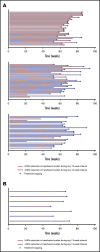
Luspatercept is an activin receptor ligand trap that has been shown to enhance late-stage erythropoiesis in animal models of β-thalassemia. A multicenter, international, phase 2 dose-finding study was initiated in adult patients with β-thalassemia, either non-transfusion-dependent thalassemia (NTDT) or transfusion-dependent thalassemia (TDT). Positive results of the phase 2 study paved the way to a randomized phase 3 clinical trial (BELIEVE) to assess the efficacy and safety of luspatercept. The BELIEVE trial is a randomized, double-blind, placebo-controlled phase 3 trial. Three hundred thirty-six patients aged ≥18 years with TDT (regularly transfused, 6-20 red blood cell units within 24 weeks before randomization) were included in the trial. Patients received luspatercept or placebo subcutaneously every 21 days for ≥48 weeks and best supportive care. Forty-eight of 224 patients (21.4%) in the luspatercept group achieved the primary end points (≥33% reduction in transfusion burden) compared with those in the placebo group (4.5%; P < .001). Moreover, more patients had a ≥33% reduction in transfusion burden during any rolling 12-week interval (70.5% vs 29.5%) or any 24-week interval (41.1% vs 2.7%) with luspatercept than with the placebo. Transfusion independence was achieved by 11% of patients in the luspatercept group. Transient adverse events were more frequent with luspatercept than with placebo, but were manageable. Luspatercept was approved by the US Food and Drug Administration in 2019 and by the European Medicines Agency in 2020. The luspatercept trial is registered on www.clinicaltrials.gov at #NCT01749540 and the BELIEVE trial at #NCT02604433.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.D.C. has served on advisory boards for BMS/Celgene, CRISPR, Novartis, Novonordisk, Sanofi/Genzyme, Silence, and Vifor. A.T.T. has provided consultancy for Abfero Pharmaceuticals Inc, Ionis Pharmaceuticals, La Jolla Pharmaceuticals, Novartis, and Protagonist Therapeutics; has received research funding from Bristol Myers Squibb, La Jolla Pharmaceuticals, Novartis, and Protagonist Therapeutics; and has received honoraria from Novartis.
Figures


References
-
- Weatherall DJ, Clegg JB. The Thalassaemia Syndromes. 4th ed. Oxford, United Kingdom: Blackwell Science; 2001.
-
- Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391(10116):155-167. - PubMed
-
- Baronciani D, Angelucci E, Potschger U, et al. Hemopoietic stem cell transplantation in thalassemia: a report from the European Society for Blood and Bone Marrow Transplantation Hemoglobinopathy Registry, 2000-2010. Bone Marrow Transplant. 2016;51(4):536-541. - PubMed
-
- Reblozyl® (luspatercept-aamt): U.S. prescribing information. Summit, NJ: Celgene Corporation; 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761136orig2lbl.... Accessed 25 September 2020.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

