Validation of a deep-learning semantic segmentation approach to fully automate MRI-based left-ventricular deformation analysis in cardiotoxicity
- PMID: 33571002
- PMCID: PMC8010548
- DOI: 10.1259/bjr.20201101
Validation of a deep-learning semantic segmentation approach to fully automate MRI-based left-ventricular deformation analysis in cardiotoxicity
Abstract
Objective: Left-ventricular (LV) strain measurements with the Displacement Encoding with Stimulated Echoes (DENSE) MRI sequence provide accurate estimates of cardiotoxicity damage related to chemotherapy for breast cancer. This study investigated an automated and supervised deep convolutional neural network (DCNN) model for LV chamber quantification before strain analysis in DENSE images.
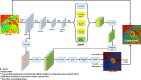
Methods: The DeepLabV3 +DCNN with three versions of ResNet-50 backbone was designed to conduct chamber quantification on 42 female breast cancer data sets. The convolutional layers in the three ResNet-50 backbones were varied as non-atrous, atrous and modified, atrous with accuracy improvements like using Laplacian of Gaussian filters. Parameters such as LV end-diastolic diameter (LVEDD) and ejection fraction (LVEF) were quantified, and myocardial strains analyzed with the Radial Point Interpolation Method (RPIM). Myocardial classification was validated with the performance metrics of accuracy, Dice, average perpendicular distance (APD) and others. Repeated measures ANOVA and intraclass correlation (ICC) with Cronbach's α (C-Alpha) tests were conducted between the three DCNNs and a vendor tool on chamber quantification and myocardial strain analysis.
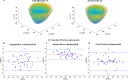
Results: Validation results in the same test-set for myocardial classification were accuracy = 97%, Dice = 0.92, APD = 1.2 mm with the modified ResNet-50, and accuracy = 95%, Dice = 0.90, APD = 1.7 mm with the atrous ResNet-50. The ICC results between the modified ResNet-50, atrous ResNet-50 and vendor-tool were C-Alpha = 0.97 for LVEF (55±7%, 54±7%, 54±7%, p = 0.6), and C-Alpha = 0.87 for LVEDD (4.6 ± 0.3 cm, 4.6 ± 0.3 cm, 4.6 ± 0.4 cm, p = 0.7).
Conclusion: Similar performance metrics and equivalent parameters obtained from comparisons between the atrous networks and vendor tool show that segmentation with the modified, atrous DCNN is applicable for automated LV chamber quantification and subsequent strain analysis in cardiotoxicity.
Advances in knowledge: A novel deep-learning technique for segmenting DENSE images was developed and validated for LV chamber quantification and strain analysis in cardiotoxicity detection.
Figures



References
-
- Bjorck J, Gomes C, Selman B, Weinberger KQ. Understanding batch normalization in 32nd conference on neural information processing systems (NeurIPS 2018). NIPS; 2018. pp. 180–4.
-
- Chen LC, Barron JT, Papandreou G, Murphy K, Yuille AL. Semantic image segmentation with task-specific edge detection using cnns and a discriminatively trained domain transform. CVPR; 2016. pp. 4545–54.
-
- Chen LC, Papandreou G, Kokkinos I, Murphy K, Yuille AL. DeepLab: semantic image segmentation with deep Convolutional nets, Atrous convolution, and fully connected CRFs. CVPR. 40; 2016. pp. 834–48. - PubMed
-
- Chen LC, Yukun Z, Papandreou G, Schroff F, Hartwig A. Encoder-decoder with Atrous separable convolution for semantic image segmentation. CVPR; 2018. pp. 801–18.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

