Venous Thromboembolism, Corticosteroids and COVID-19: A Systematic Review and Meta-Analysis
- PMID: 33571009
- PMCID: PMC7883150
- DOI: 10.1177/1076029621993573
Venous Thromboembolism, Corticosteroids and COVID-19: A Systematic Review and Meta-Analysis
Abstract
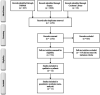
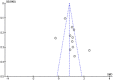
The novel coronavirus disease 2019 (COVID-19) predisposes patients to venous thromboembolism (VTE) due to risk factors, severe infection, and severe inflammatory responses. The objective is to determine the risk of developing VTE after corticosteroid administration during COVID-19 treatment. Using PRISMA reporting guidelines, a review was conducted from inception until 20 September 2020 with MESH terms including "venous thromboembolism" and "covid-19," using MEDLINE, Scopus, CINAHL Plus, and WHO Global Database. The inclusion criteria included studies with COVID-19 patients aged 18 years and older with VTE diagnosed by duplex ultrasonography or computed tomography pulmonary angiography (CTPA). Exclusion criteria were studies with non COVID-19 patients and non-VTE patients aged less than 18 years. Quality appraisal was conducted of included studies using the Newcastle-Ottawa Scale (NOS). A random-effect model using 95% confidence intervals, and significance of findings was assessed using Review Manager V5.4.We included 12 observational studies with 2801 patients (VTE n = 434; non-VTE; n = 2367). Patients had a higher risk of presenting with VTE when being administered corticosteroids during treatment of COVID-19 (RR = 1.39, 95% CI = 1.10 to 1.77, I2 = 0%). A positive effect size was found (SMD = 1.00, 95% CI = 0.67 to 1.32, I2 = 85%) for D-dimer laboratory values (µg/mL) in the VTE group. While critically ill COVID-19 patients are more likely to require corticosteroid treatment, it may be associated with increased risk of VTE, and poor clinical prognosis. Risk assessment is warranted to further evaluate patients as case-by-case in reducing VTE and worsening clinical outcomes.
Keywords: coronavirus; deep vein thrombosis; mortality; pulmonary embolism; venous thromboembolism.
Conflict of interest statement
Figures




Similar articles
-
A systematic review and meta-analysis of incidence, prognosis, and laboratory indicators of venous thromboembolism in hospitalized patients with coronavirus disease 2019.J Vasc Surg Venous Lymphat Disord. 2021 Sep;9(5):1099-1111.e6. doi: 10.1016/j.jvsv.2021.01.012. Epub 2021 Jan 30. J Vasc Surg Venous Lymphat Disord. 2021. PMID: 33529719 Free PMC article.
-
COVID-19 Infection in Critically Ill Patients Carries a High Risk of Venous Thrombo-embolism.Eur J Vasc Endovasc Surg. 2021 Apr;61(4):628-634. doi: 10.1016/j.ejvs.2020.12.015. Epub 2020 Dec 23. Eur J Vasc Endovasc Surg. 2021. PMID: 33583710 Free PMC article.
-
Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients.Thromb Res. 2020 Dec;196:308-312. doi: 10.1016/j.thromres.2020.09.017. Epub 2020 Sep 15. Thromb Res. 2020. PMID: 32977128 Free PMC article.
-
D-Dimer, Fibrinogen, and IL-6 in COVID-19 Patients with Suspected Venous Thromboembolism: A Narrative Review.Vasc Health Risk Manag. 2020 Nov 13;16:455-462. doi: 10.2147/VHRM.S280962. eCollection 2020. Vasc Health Risk Manag. 2020. PMID: 33223833 Free PMC article. Review.
-
Venous Thromboembolism in Patients Discharged after COVID-19 Hospitalization.Semin Thromb Hemost. 2021 Jun;47(4):362-371. doi: 10.1055/s-0041-1727284. Epub 2021 Apr 23. Semin Thromb Hemost. 2021. PMID: 33893631 Clinical Trial.
Cited by
-
The effectiveness of glucocorticoid treatment in post-COVID-19 pulmonary involvement.Pneumonia (Nathan). 2024 Feb 5;16(1):2. doi: 10.1186/s41479-023-00123-7. Pneumonia (Nathan). 2024. PMID: 38311783 Free PMC article.
-
The interplay between inflammation and thrombosis in COVID-19: Mechanisms, therapeutic strategies, and challenges.Thromb Update. 2022 Aug;8:100117. doi: 10.1016/j.tru.2022.100117. Epub 2022 Jul 9. Thromb Update. 2022. PMID: 38620713 Free PMC article. Review.
-
The Important Role of Interleukin-2 in COVID-19.J Immunol Res. 2023 Aug 22;2023:7097329. doi: 10.1155/2023/7097329. eCollection 2023. J Immunol Res. 2023. PMID: 37649897 Free PMC article. Review.
-
A Prospective Cohort Study of COVID-19: Evaluation of the Early Role of IL-1 and IL-6 Antagonists in Improving the Outcome of the Illness and Reduction in the Risk of Death.Healthcare (Basel). 2023 Apr 3;11(7):1025. doi: 10.3390/healthcare11071025. Healthcare (Basel). 2023. PMID: 37046952 Free PMC article.
-
Dexamethasone Modulates the Cytokine Response but Not COVID-19-Induced Coagulopathy in Critically Ill.Int J Mol Sci. 2023 Apr 14;24(8):7278. doi: 10.3390/ijms24087278. Int J Mol Sci. 2023. PMID: 37108440 Free PMC article.
References
-
- Liu X, Liu C, Chen X, Wu W, Lu G. Comparison between Caprini and Padua risk assessment models for hospitalized medical patients at risk for venous thromboembolism: a retrospective study. Interact Cardiovasc Thorac Surg. 2016;23(4):538–543. - PubMed
-
- Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol [Internet]. 2020;7(5): e632–e363 https://pubmed.ncbi.nlm.nih.gov/32278361 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

