A novel murine muscle loading model to investigate Achilles musculotendinous adaptation
- PMID: 33571057
- PMCID: PMC8262782
- DOI: 10.1152/japplphysiol.00638.2020
A novel murine muscle loading model to investigate Achilles musculotendinous adaptation
Abstract
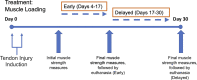
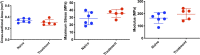
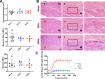
Achilles tendinopathy is a debilitating condition affecting the entire spectrum of society and a condition that increases the risk of tendon rupture. Effective therapies remain elusive, as anti-inflammatory drugs and surgical interventions show poor long-term outcomes. Eccentric loading of the Achilles muscle-tendon unit is an effective physical therapy for treatment of symptomatic human tendinopathy. Here, we introduce a novel mouse model of hindlimb muscle loading designed to achieve a tissue-targeted therapeutic exercise. This model includes the application of tissue (muscle and tendon)-loading "doses," coupled with ankle dorsiflexion and plantarflexion, inspired by human clinical protocols. Under computer control, the foot was rotated through the entire ankle joint range of motion while the plantar flexors simultaneously contracted to simulate body mass loading, consistent with human therapeutic exercises. This approach achieved two key components of the heel drop and raise movement: ankle range of motion coupled with body mass loading. Model development entailed the tuning of parameters such as footplate speed, number of repetitions, number of sets of repetitions, treatment frequency, treatment duration, and treatment timing. Initial model development was carried out on uninjured mice to define a protocol that was well tolerated and nondeleterious to tendon biomechanical function. When applied to a murine Achilles tendinopathy model, muscle loading led to a significant improvement in biomechanical outcome measures, with a decrease in cross-sectional area and an increase in material properties, compared with untreated animals. Our model facilitates the future investigation of mechanisms whereby rehabilitative muscle loading promotes healing of Achilles tendon injuries.NEW & NOTEWORTHY We introduce a novel mouse model of hindlimb muscle loading designed to achieve a tissue-targeted therapeutic exercise. This innovative model allows for application of muscle loading "doses," coupled with ankle dorsiflexion and plantarflexion, inspired by human loading clinical treatment. Our model facilitates future investigation of mechanisms whereby rehabilitative muscle loading promotes healing of Achilles tendon injuries.
Keywords: Achilles; eccentric; muscle loading; preclinical; tendinopathy.
Figures


Similar articles
-
Efficacy of heel lifts versus calf muscle eccentric exercise for mid-portion Achilles tendinopathy (the HEALTHY trial): study protocol for a randomised trial.J Foot Ankle Res. 2019 Mar 21;12:20. doi: 10.1186/s13047-019-0325-2. eCollection 2019. J Foot Ankle Res. 2019. PMID: 30949243 Free PMC article.
-
Functional Ankle Range of Motion but Not Peak Achilles Tendon Force Diminished With Heel-Rise and Jumping Tasks After Achilles Tendon Repair.Am J Sports Med. 2021 Jul;49(9):2439-2446. doi: 10.1177/03635465211019436. Epub 2021 Jun 11. Am J Sports Med. 2021. PMID: 34115525 Free PMC article.
-
Investigating the Effects of Knee Flexion during the Eccentric Heel-Drop Exercise.J Sports Sci Med. 2015 May 8;14(2):459-65. eCollection 2015 Jun. J Sports Sci Med. 2015. PMID: 25983597 Free PMC article.
-
[Insertional Achilles tendinopathy : Differentiated diagnostics and therapy].Unfallchirurg. 2017 Dec;120(12):1044-1053. doi: 10.1007/s00113-017-0415-1. Unfallchirurg. 2017. PMID: 28980027 Review. German.
-
Foundational Principles and Adaptation of the Healthy and Pathological Achilles Tendon in Response to Resistance Exercise: A Narrative Review and Clinical Implications.J Clin Med. 2022 Aug 12;11(16):4722. doi: 10.3390/jcm11164722. J Clin Med. 2022. PMID: 36012960 Free PMC article. Review.
Cited by
-
Early Tendon Morphology as a Biomarker of Long-term Patient Outcomes After Surgical Repair of Achilles Tendon Rupture: A Prospective Cohort Study.Orthop J Sports Med. 2023 Nov 6;11(11):23259671231205326. doi: 10.1177/23259671231205326. eCollection 2023 Nov. Orthop J Sports Med. 2023. PMID: 37941888 Free PMC article.
-
Tendon regeneration deserves better: focused review on In vivo models, artificial intelligence and 3D bioprinting approaches.Front Bioeng Biotechnol. 2025 Apr 25;13:1580490. doi: 10.3389/fbioe.2025.1580490. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40352349 Free PMC article. Review.
-
Optogenetic-induced muscle loading leads to mechanical adaptation of the Achilles tendon enthesis in mice.Sci Adv. 2023 Jun 23;9(25):eadf4683. doi: 10.1126/sciadv.adf4683. Epub 2023 Jun 23. Sci Adv. 2023. PMID: 37352350 Free PMC article.
-
Leveraging in vivo animal models of tendon loading to inform tissue engineering approaches.Front Bioeng Biotechnol. 2024 Oct 7;12:1449372. doi: 10.3389/fbioe.2024.1449372. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39434716 Free PMC article. Review.
-
Optogenetic-Induced Muscle Loading Leads to Mechanical Adaptation of the Achilles Tendon Enthesis in Mice.bioRxiv [Preprint]. 2023 May 2:2023.04.11.536376. doi: 10.1101/2023.04.11.536376. bioRxiv. 2023. Update in: Sci Adv. 2023 Jun 23;9(25):eadf4683. doi: 10.1126/sciadv.adf4683. PMID: 37090593 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

