Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with high-titer convalescent plasma
- PMID: 33571168
- PMCID: PMC8026191
- DOI: 10.1172/jci.insight.143196
Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with high-titer convalescent plasma
Abstract
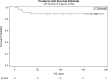
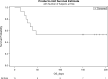
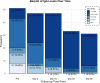
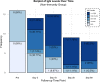
Here, we report on a phase IIa study to determine the intubation rate, survival, viral clearance, and development of endogenous Abs in patients with COVID-19 pneumonia treated with convalescent plasma (CCP) containing high levels of neutralizing anti-SARS-CoV-2 Abs. Radiographic and laboratory evaluation confirmed all 51 treated patients had COVID-19 pneumonia. Fresh or frozen CCP from donors with high titers of neutralizing Abs was administered. The nonmechanically ventilated patients (n = 36) had an intubation rate of 13.9% and a 30-day survival rate of 88.9%, and the overall survival rate for a comparative group based on network data was 72.5% (1625/2241). Patients had negative nasopharyngeal swab rates of 43.8% and 73.0% on days 10 and 30, respectively. Patients mechanically ventilated had a day-30 mortality rate of 46.7%; the mortality rate for a comparative group based on network data was 71.0% (369/520). All evaluable patients were found to have neutralizing Abs on day 3 (n = 47), and all but 1 patient had Abs on days 30 and 60. The only adverse event was a mild rash. In this study on patients with COVID-19 disease, we show therapeutic use of CCP was safe and conferred transfer of Abs, while preserving endogenous immune response.
Keywords: COVID-19; Immunotherapy.
Conflict of interest statement
Figures
References
-
- WHO. Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ Updated February 11, 2021. Accessed February 11, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

